Article Text
Abstract
Non-cardiac comorbidity complicates heart failure care and is prevalent in one form or another for the majority of elderly patients with heart failure. This wide range of comorbidities, which includes respiratory comorbidities, renal dysfunction, anaemia, arthritis, cognitive dysfunction and depression, contributes to the progression of the disease and may alter the response to treatment. Polypharmacy is inevitable in these patients. Cardiologists and other physicians caring for patients with chronic heart failure (CHF) need to be vigilant to comorbid conditions that may complicate the care of these patients. Future trials should focus on optimal strategies for the comprehensive management of the elderly patients with CHF with multiple comorbidities rather than the isolated effects of single drugs in younger patients with few or no comorbidities.
- BNP, brain natriuretic peptide
- CHF, chronic heart failure
- COPD, chronic obstructive pulmonary disease
- CPAP, continuous positive airway pressure
- CSA, central sleep apnoea
- HF, heart failure
- NYHA, New York Heart Association
- OSA, obstructive sleep apnoea
- rHuEPO, recombinant human erythropoietin
- SDB, sleep-disordered breathing
- TNF, tumour necrosis factor
Statistics from Altmetric.com
- BNP, brain natriuretic peptide
- CHF, chronic heart failure
- COPD, chronic obstructive pulmonary disease
- CPAP, continuous positive airway pressure
- CSA, central sleep apnoea
- HF, heart failure
- NYHA, New York Heart Association
- OSA, obstructive sleep apnoea
- rHuEPO, recombinant human erythropoietin
- SDB, sleep-disordered breathing
- TNF, tumour necrosis factor
Chronic heart failure (CHF) is the leading diagnosis at hospital discharge for elderly patients. In these elderly patients, CHF is often accompanied by a range of comorbidities that play an integral role in its progression and response to treatment. Comorbidity is defined as a chronic condition that coexists in an individual with another condition that is being described. A distinction is made between non-cardiac comorbidities and cardiac conditions that are directly related to the presence of CHF such as arrhythmias as well conditions that predate and contribute to its aetiology such as hypertension, diabetes mellitus and hyperlipidaemia. This article will focus largely on non-cardiac comorbidities in CHF.
EPIDEMIOLOGY OF NON-CARDIAC COMORBIDITIES IN CHF
Previous data on the presence and effect of comorbidities on CHF were derived from clinical trials and geographically limited studies of relatively small numbers of patients such as the Framingham cohort.1 However, data from CHF trials are not reflective of the real-world situation as these are largely derived from younger patients with few or no comorbidities. Lately, studies have utilised databases to examine the impact of comorbidity in larger groups of elderly patients with CHF. Utilising data from 27 477 Scottish morbidity records listing CHF, Brown and Cleland2 reported that 11.8% of CHF admissions were associated with chronic airways obstruction, 8.3% with chronic or acute renal failure and 5.3% with cerebrovascular accident. In the US, the National Heart Failure project, an effort by the Centers for Medicare and Medicaid Services, found that comorbidity was common among 34 587 Medicare elderly patients aged ⩾65 years, hospitalised with a principal diagnosis of CHF.3 About a third (32.9%) had chronic obstructive pulmonary disease (COPD), 18% had a history of stroke and 9.2% had dementia. More recently, Braunstein and colleagues4 reported the findings of a cross-sectional analysis of 122 630 individuals aged ⩾65 years with CHF identified through a 5% random sample of all US Medicare beneficiaries. Nearly 40% of patients with CHF had ⩾5 non-cardiac comorbidities, and this group accounted for 81% of the total inpatient hospital days experienced by patients with CHF. The top 10 most common non-cardiac conditions were COPD/bronchiectasis (26%), osteoarthritis (16%), chronic respiratory failure or other lower respiratory disease excluding COPD/bronchiectasis (14%), thyroid disease (14%), Alzheimer’s disease/dementia (9%), depression (8%), chronic renal failure (7%), asthma (5%), osteoporosis (5%) and anxiety (3%). The risk of hospitalisation and potentially preventable hospitalisations strongly increased with the number of chronic conditions (fig 1). After controlling for demographic factors and other diagnoses, comorbidities that were associated consistently with higher risks for CHF hospitalisations and mortality included COPD/bronchiectasis, renal failure, diabetes, depression and lower respiratory diseases. Several reasons may explain why older patients with CHF with greater comorbidity may experience more adverse events that lead to preventable hospitalisations. These include underutilisation of effective CHF treatments in the presence of other conditions because of safety concerns (eg, use of β-blockers in asthma or ACE inhibitors in renal insufficiency), patient non-adherence to or inability to recall complex medication regimens, inadequate postdischarge care, failed social support and failure to promptly seek medical attention during symptom recurrence. Psychological stress from chronically poor health may also predispose to bad outcomes. Finally, elderly patients with multiple comorbidities and polypharmacy are also susceptible to poor coordination of care and are also at an increased risk for experiencing adverse drug reactions from drug–drug interactions. The association between comorbidity and healthcare costs has also been examined in a Medicare healthcare expenditure study.5 Patients with CHF having expensive comorbidities included those with chronic pulmonary disease (33% of patients, mean total annual expenditure $23 104 (£13 355, €17 442) per patient), renal disease (8% of patients, mean total annual expenditure $33 014 (£19 083, €24 924) per patient), rheumatological disease (5% of patients, mean total annual expenditure, $20 527 (£11 865, €15 497) per patient) and dementia (15% of patients, mean total annual expenditure, $26 263 (£15 181, €19 827) per patient).
Impact of non-cardiac comorbidity burden on the annual probability of a Medicare beneficiary with chronic heart failure (CHF) experiencing a hospitalisation due to any cause, a preventable hospitalisation or a preventable hospitalisation due to CHF. p<0.001 for linear trend for all outcomes. ACSC, ambulatory care sensitive conditions. Reprinted with permission from Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among medicare beneficiaries with chronic heart failure. J Am Coll Cardiol 2003;42:1226–33, copyright 2003, with permission from The American College of Cardiology Foundation.4
MANAGEMENT OF SPECIFIC NON-CARDIAC COMORBIDITIES
We shall now discuss specifically how best to manage CHF in patients with the following common non-cardiac comorbidities in CHF: (1) respiratory comorbidities; (2) renal dysfunction; (3) anaemia; (4) cognitive dysfunction; (5) depression; (6) arthritis.
RESPIRATORY DISORDERS
The interaction between CHF and concomitant respiratory disease is common and important. Many patients with CHF are misdiagnosed as having air flow obstruction on the basis of overlapping symptoms (and vice versa). In patients presenting to the casualty department with dyspnoea, both brain natriuretic peptide (BNP) and N-terminal prohormone BNP have been shown to be useful in improving the diagnostic accuracy of CHF.6 Recently, in a controlled study of the use of both BNP and N-terminal prohormone in 306 patients with suspected CHF referred to rapid access CHF clinics,7 both tests proved useful, in particular when the results were normal. When both CHF and respiratory disorders coexist, it may be important to quantify the relative contribution of cardiac and pulmonary components to the disability. Exercise testing with simultaneous gas exchange or blood gas measurements may be helpful in this regard. Optimum assessment and management of these patients need careful consideration of the possibility that cardiac and respiratory disease may coexist in the individual patient.
Obstructive airways disease
β-blockers are deemed to be contraindicated in patients with CHF and air flow obstruction. In practice, because of the overwhelming benefits of these agents in CHF, many patients with fixed or limited airways reversibility are given them, and they tolerate them surprisingly well. Whether β-1-selective agents offer advantages over non-selective agents such as carvedilol is not clear.8,9 Cardioselective β-blockers given in mild to moderate reversible airway disease do not produce adverse respiratory effects in the short term. Given their demonstrated benefit in heart failure (HF), these agents should not be withheld from such patients, but long-term safety (especially, their impact during an acute exacerbation) still needs to be established.
Sleep-disordered breathing
There has been increasing interest in the role of sleep-disordered breathing (SDB) in patients with CHF. Although often neglected in the clinical practice and CHF literature for many years, SDB in patients with CHF has recently been gaining recognition on the basis of its clinical relevance10,11 and the subject has recently been reviewed by Cormican and Williams.10 It is of significance that both CHF and SDB constitute multisystem disorders involving respiratory, cardiovascular and neurohumoral axes. Two major types of SDB observed in CHF are obstructive sleep apnoea (OSA) and central sleep apnoea (CSA). These two types of SDB operate through different pathophysiological mechanisms, although they can coexist and interact.10 OSA results from complete or partial collapse of a normal pharynx. Struggling to breathe against the throat causes generation of negative intrathoracic pressure, leading to marked loading of the ventricles. By contrast, CSA results from either a reduction in central respiratory drive or instability in feedback control of the central respiratory centre. OSA, a likely risk factor for cardiovascular disease, may contribute to both the development and progression of CHF. Moreover, OSA shares with CHF many aspects of deranged neurohumoral and immunological function. CSA, on the other hand, may be a consequence of CHF, but when present, may increase the risk of arrhythmias and impair prognosis.10
The prevalence of SDB is high in CHF. Up to 40% of patients with CHF have CSA and 11% have OSA.10 In the most recent data, SDB is common even in stable outpatients with CHF.12 It should be noted that the majority of patients with CHF having SDB remain undiagnosed. This is due to both a limitation in awareness as well as the limited availability of sleep laboratories. Although attended nocturnal polysomnography in a sleep laboratory is the preferred diagnostic method,13 home-based unattended sleep studies that combine continuous pulse rate and recording the oxyhaemoglobin saturation have been used as screening tools.9 It has been suggested that patients with CHF should also be routinely asked simple screening questions such as “Do you snore?” or “Do you fall asleep during the day?” to help identify potential patients with SDB.
Treatment of SDB
Haemodynamic improvement following medical treatment is often associated with a significant improvement in the nocturnal breathing pattern. In patients with persistent SDB, especially when it is associated with marked desaturations and refractory CHF, a specific treatment of the breathing disorder should be considered.
Nocturnal nasal continuous positive airway pressure
There have been a number of randomised controlled trials of continuous positive airway pressure (CPAP) therapy in patients with CHF and OSA or CSA. In terms of OSA, there have been at least four small-scale studies,10 which consistently show that CPAP reverses obstructive apnoea and improves oxygenation at night. CPAP also lowers sympathetic activity and improves left ventricular ejection fraction of up to 9%. There are a similar number of randomised controlled trials of CPAP in CSA when applied for at least 1–3 months with similar benefits of reduced apnoea–hypopnoea index, improvement in ejection fraction (6.5–8.6%) and functional class.10 It should be emphasised that the sample sizes of these trials are small and of short follow-up and consequently, it was only the study by Sin and colleagues14 that reported a risk reduction in combined mortality-cardiac transplantation rate in CHF with CSA but not in patients without CSA. Nevertheless, these findings prompted the Canadian Continuous Positive Airway Pressure trial, the results of which have just been published.15 The study was terminated early in May 2004 when there was a dramatic drop in the expected rate of primary events caused largely by the introduction of β-blockers. This resulted in the study being statistically underpowered. Only 258 of the anticipated 408 patients were randomised with a mean follow-up of 24 months. Despite a reported 50% reduction in the apnoea–hypopnoea index (39.7–18.6 apnoeas per hour, p<0.001), an improvement in both left ventricular systolic function (p = 0.007), and an increase in 6-minute walking distance (an increase of 20 m vs −0.8 m, p = 0.016), the transplant-free survival and rates of CHF-related hospitalisations were identical in the CPAP and control groups. Of concern was the observation of an early divergence of events favouring the control group during the first 18 months (p = 0.02), which then reversed and favoured the CPAP group after 18 months. The mechanism for this observation is a subject of debate and has not been fully defined. Thus, although CPAP alleviated CSA and improved cardiac function, its effectiveness in improving transplant-free survival remains in question.
OTHER TREATMENT STRATEGIES FOR SDB IN CHF
The adaptive servo-ventilator has been designed for the treatment of CSA in patients with CHF and provides a baseline degree of ventilatory support, where the patient’s ventilation is servo-controlled to maintain the ventilation at 90% of the long-term average.16 In a crossover trial conducted in 14 patients in New York Heart Association (NYHA) II–III, this approach provided an additional 83% reduction in central apnoeas when compared with nasal CPAP, and was also more easily tolerated.
Oxygen supplementation has been tested in CSA although the rationale is not entirely clear. It has been hypothesised that O2 may stabilise the breathing by removing any enhancement of the hypercapnic chemoreflex and attenuate any independent influence of hypoxic chemoreflex activation on the hyperventilatory response. In a crossover study of patients with CHF in NYHA functional class IV, single-night treatments with O2 supplements and nasal CPAP were equally effective in decreasing the apnoea–hypopnoea index, arousal index and degree of desaturation.17 Although a clear advantage of O2 is the greater acceptability by the patients, data on the effect of nocturnal O2 treatment on medium-term clinical and functional end points are not currently available.
Interesting results have been shown with oral theophylline, which, in the short term, resulted in a 50% reduction in apnoea–hypopnoea index and related arousals.18 Among the potential mechanisms, an increased inotropic effect and a direct stimulation (and stabilisation) of the central ventilatory drive have been invoked as playing a role in reducing the respiratory events. Data on long-term efficacy and safety are not available at present.
Finally, overdrive pacing has also been shown to elicit a 60% reduction of both central and obstructive apnoeas in a small group of highly selected patients with symptomatic sinus bradyarrhythmias and coexisting sleep apnoea.19 Although interesting, these results should be considered preliminary. Long-term studies are necessary to determine their impact on the natural history of left ventricular systolic dysfunction and on CHF-related morbidity and mortality.
RENAL DYSFUNCTION IN CHF
The close relationship between cardiovascular and renal function in normal physiology is also apparent in the disease. As a consequence of accelerated atherosclerotic coronary artery disease, concomitant hypertension and fluid retention, patients with primary renal disease are at high risk of CHF. Conversely, many patients with CHF have evidence of kidney dysfunction in the absence of intrinsic renal disease. About 40% of patients with CHF have chronic kidney disease, defined as a serum creatinine level of ⩾133 μmol/l or a creatinine clearance rate of <60 ml/min.20 The observed low glomerular filtration rate in CHF is a consequence of diminished cardiac output, with decreased renal perfusion and intrarenal vasoconstriction accompanied by sodium and water retention. Indeed, given the relationship between renal function and cardiac output, renal dysfunction is surprisingly an adverse prognostic marker, and also a strong predictor of poor outcome in HF than functional class.20 Multiple theories have been offered to explain the excess risks in patients with CHF with renal dysfunction including more advanced coronary atherosclerosis. However, recent data from 6427 patients with CHF with documented coronary angiography showed that the adverse prognostic influence of renal dysfunction was independent of atherosclerotic burden and left ventricular systolic function.20
Heart failure therapy in patients with CHF with renal dysfunction
The effects of CHF drugs on patients with CHF with renal dysfunction have not been well studied. Patients with renal hypoperfusion or intrinsic renal disease show an impaired response to diuretics and ACE inhibitors21 and are at an increased risk of adverse effects during treatment with digitalis. Most CHF trials have studied patients with normal renal function with only a few reporting subgroup analyses of patients with renal dysfunction. In the Cardiac Insufficiency Bisoprolol Study, those with moderate to severe renal failure showed a similar benefit on mortality and hospitalisation from bisoprolol treatment to those with normal renal function.22 Perhaps the most impressive effect of a β-blocker in CHF and end-stage renal failure was recently reported by Cice et al.23 A total of 114 dialysis patients with dilated cardiomyopathy were randomised to receive either carvedilol or a placebo in addition to standard therapy. At 2 years, the carvedilol group had smaller cavity diameters in both systole and diastole and had higher ejection fractions. By 2 years, 51.7% of the patients in the carvedilol group had died, whereas 73.2% in the placebo group had died. There were significantly fewer cardiovascular deaths and fewer hospital admissions among the patients receiving carvedilol. All these data strongly support the use of such drugs in patients with CHF with chronic kidney disease. In view of the extremely high cardiovascular morbidity and mortality in chronic kidney disease and end-stage renal disease, there is clearly a need for routine use of such cardioprotective agents in patients with cardiac damage. Unfortunately, in the “real patient” population, prescription rates for such CHF drugs are inversely related to renal function.20
As the renal vasoconstriction that develops in the setting of reduced cardiac output depends on angiotensin II, treatment with an ACE inhibitor or angiotensin-receptor blocker commonly leads to an (generally, clinically unimportant) increase in the serum creatinine concentration. Generally, these slight rises in serum creatinine levels are reversible and are only infrequently the cause of drug discontinuation. Most patients with CHF tolerate mild to moderate degrees of functional renal impairment without difficulty. However, if the serum creatinine increases >220 μmol/l, the presence of renal insufficiency can severely limit the efficacy and enhance the toxicity of established treatments. An arbitrary creatinine cut-off value to define renal insufficiency (serum creatinine >220 μmol/l) for spironolactone has been suggested in published guidelines.11 However, this may not be appropriate in the elderly because of the competing age-related decline in creatinine as a result of decline in muscle mass and rise in creatinine as a result of decline in glomerular filtration rate. Indeed, in a prescription linked study, Juurlink and colleagues24 found that immediately after the publication of the Randomized Aldactone Evaluation Study, the prescription rate in Canada rose sharply and that this was associated with an increase in the rate of admission for hyperkalaemia, from 2.4 per 1000 patients in 1994 to 11 per 1000 patients in 2001 (p<0.001), and the associated mortality rose from 0.3 per 1000 to 2 per 1000 patients (p<0.001). However, it should be noted that there were several differences in this real-patient population study from the cohort in the Randomized Aldactone Evaluation Study trial. These older patients received a higher dose of spironolactone without close attention to serum creatinine and follow-up. Similar findings have also been described elsewhere. In the study of Medicare beneficiaries aged ⩾65 years discharged after hospitalisation for CHF, spironolactone was prescribed to 22.8% of patients with serum potassium ⩾5 mmol/l, to 14.1% with a serum creatinine value ⩾220 μmol/l and to 17.3% with severe renal dysfunction (glomerular filtration rate <30 ml/min/1.73 m2).25 In multivariate analysis, factors associated with such prescribing patterns included advanced age and non-cardiovascular morbidities. Clearly, there is a need for greater vigilance and care to be given to frequent monitoring of electrolytes and renal parameters in patients with CHF with renal dysfunction.
ANAEMIA
Anaemia has recently been recognised as an important comorbid condition and potentially novel therapeutic target in patients with CHF. Anaemia is common in patients with HF, with a prevalence ranging from 4% to 55%.26 Reasons for this wide variation include differences in the population with CHF studied, in study methods, and in the definition of anaemia used. Although the most commonly accepted definition of anaemia is that of the World Health Organization (haemoglobin <13 g/dl in men and <12 g/dl in women), studies have varied considerably in the criteria used to classify patients as anaemic. In general, the prevalence of anaemia is greater in less-selected populations (such as insurance claims data) and lower in highly selected populations such as patients enrolled in clinical trials. Anaemia appears to be more common in patients with more severe disease, with a reported prevalence in patients with NYHA functional class IV populations as high as 79%. Multiple potential mechanisms of interaction exist between anaemia and the clinical syndrome of HF. CHF is a disease of the elderly, a population where the prevalence of anaemia is high irrespective of cardiac status. Multiple comorbid conditions are common in patients with CHF, in particular, renal insufficiency, which is closely associated with the development of anaemia. Other potential contributing factors include haemodilution, proinflammatory cytokines, malnutrition due to right-sided HF and decreased perfusion to the bone marrow. With respect to haemodilution, expansion of plasma volume is a characteristic of the CHF syndrome, and, therefore, some anaemia may be dilutional rather than due to a true decrease in red blood cell mass. In a study of 37 patients with CHF, we found that, true anaemia (ie, a decrease in red blood cell mass) was present in 54%, and haemodilution was present in 46%. Notably, in this study, both haemodilution and true anaemia were associated with adverse survival, with the worst survival seen in patients with haemodilution.27 In reality, it is likely that several of these mechanisms are active simultaneously, and that anaemia in HF is the result of a complex interaction between cardiac performance, neurohormonal and inflammatory activation, renal function and bone marrow responsiveness. A prospective ongoing study including both specialty and community sites, the Study of Anaemia in a Heart Failure Population registry, is evaluating the prevalence, aetiologies and mechanisms of anaemia in a broad population of patients with CHF.
The association of anaemia with adverse clinical outcomes in CHF has led to substantial interest in anaemia as a potential therapeutic target. Preliminary data involving small groups of patients with HF suggest that treatment of anaemia may result in a significant symptomatic improvement in HF.27,28 We have previously evaluated the effect of 3 months of erythropoietin treatment on exercise capacity in single-blind placebo-controlled study of 26 patients with anaemia and NYHA functional class III–IV HF.28 This study demonstrated significant improvements in mean (SD) peak oxygen consumption with erythropoietin treatment (from 11 (0.8) to 12.7 (2.8) in the recombinant human erythropoietin (rHuEPO)-treated patients (p<0.05) vs no significant change in the control patients). A significant correlation was observed between increases in haemoglobin with rHuEPO treatment and increased peak oxygen consumption. Notably, the improvement in exercise performance with rHuEPO treatment was observed whether the anaemia was found to be from decreased red blood cell mass or from haemodilution. These studies used rHuEPO in a regimen similar to the one used in patients with end-stage renal disease. Newer erythropoietin analogs have been developed (such as darbepoetin α) that have a longer half-life and require less frequent administration, potentially making them more attractive for CHF treatment. It should also be noted that aggressive treatment of anaemia may also be associated with an increased risk of hypertension or thrombosis. Multiple ongoing studies will provide definitive data on the balance of risks and benefits of anaemia treatment in chronic HF. The Study of Anaemia Heart Failure Trial study is an ongoing double-blind, placebo-controlled, randomised trial of about 300 patients with CHF with anaemia (defined as having a haemoglobin level <12 g/dl) who will be randomised to treatment (subcutaneous injections every 2 weeks for 1 year) with darbepoetin α or placebo. Exercise treadmill tests will be performed at baseline and again at 13 and 27 weeks. Change in functional status will be the main end point.
At present, there is insufficient data to make a general recommendation for aggressive treatment of anaemia in patients with HF. A diagnostic evaluation for potentially reversible causes of anaemia (such as iron deficiency or occult blood loss) and subsequent treatment, if identified, is appropriate in all patients. Although pilot data on the treatment of anaemic patients with HF with erythropoietin analogs are promising, the studies published thus far have been significantly limited by very small sample size, lack of blinding and the use of subjective end points. Treatment of mild anaemia with erythropoietin analogs can, thus, not be considered a proven treatment for CHF based on currently available data, and the results of larger, more carefully controlled clinical trials will be required before such treatment could be considered a viable therapy.
COGNITIVE DYSFUNCTION
An abnormal prevalence of cognitive dysfunction ranging from 35% to >50% has been described among patients with CHF.29 Reduced cardiac output from CHF may further compromise cerebral blood flow in a patient with borderline perfusion of the cerebrum. Additionally, CHF is largely driven by vascular disease and cerebrovascular disease is an important contributor to multi-infarct dementia. Among older patients with CHF, cognitive dysfunction has been associated with a fivefold increase in the risk of mortality and a sixfold increase in the probability of dependence for the activities of daily living.30 Reducing the burden of cognitive dysfunction might allow substantial gains in terms of survival rates, quality of life and resource consumption. However, no interventions are yet known to improve cognitive performance largely because of the incomplete knowledge about the pathophysiology of cognitive dysfunction in these patients. It should be noted that measures of cognitive function have rarely been used in CHF trials, unlike hypertension trials such as Systolic Hypertension in Europe and Study on Cognition and Prognosis in the Elderly. Given the consistent reporting of impaired cognitive function in cross-sectional studies of patients with CHF, perhaps this should be considered as an endpoint for future trials of HF pharmacotherapy.
DEPRESSION AND CHF
The prevalence rates of depression in patients with CHF range from 13% to 77%.31 Depression is a graded, independent risk factor for several adverse outcomes, including decreased compliance with treatment recommendations, increases in healthcare costs, hospital admissions and mortality rates. Cognitive-behavioural therapy is the preferred psychological treatment. Cognitive-behavioural therapy emphasises the reciprocal interactions among physiology, environmental events, thoughts and behaviours, and how these may be altered to produce changes in mood and behaviour. Pharmacologically, the selective serotonin reuptake inhibitors are recommended, whereas the tricyclic antidepressants are not recommended for depression in patients with congestive HF.32 Consideration should also be given to potential interactions between antidepressant medications and those commonly used in the treatment of CHF. For example, the tricyclics, as well as fluoxetine, paroxetine and sertraline, have been reported to interact with warfarin to increase prothrombin time. The combination of a selective serotonin reuptake inhibitor with cognitive-behavioural therapy is often the most effective treatment.
ARTHRITIS
In the study by Braunstein and colleagues,4 arthritis is one of the top 10 non-cardiac comorbidities in CHF and its treatment influences HF status. Both non-steroidal anti-inflammatory drugs and cyclo-oxygenase-2 selective inhibitors are associated with potentially significant cardiovascular adverse effects in CHF.33 Sodium and water retention with these agents may adversely affect volume status partly because of activation of vasodilator prosatglandins such as E2 and I2.33 The premature suspension of the Alzheimer’s Disease Anti-inflammatory Prevention and the Adenoma Prevention with Celecoxib trials has prompted intense review of the cardiovascular safety profile of cyclo-oxygenase-2 inhibitors. With regards to non-steroidal anti-inflammatory drugs, if treatment cannot be avoided, educating the patient is important and it is recommended that the lowest dose be used and for the shortest duration possible with close monitoring of CHF status and renal function.
The role of the prostaglandin inhibitor, aspirin, in attenuating the beneficial effects of renin-angiotensin blockade in CHF is highly controversial. Although a systematic review of six large ACE inhibitor trials reported a non-significant negative interaction between ACE inhibitors and aspirin (p = 0.007), the trial in that systematic review included very few patients with serum creatinine >175 μmol/l.34 On the other hand, in studies that have included patients with moderate or severe renal insufficiency, many of whom were treated with lower doses of ACE inhibitors and higher doses of aspirin than employed in the meta-analysed trials, have reported a negative interaction.20 The dose of aspirin may matter.35 It should be noted that although the Warfarin and Antiplatelet Therapy in Chronic Heart Failure trial reported excess CHF hospitalisations in aspirin-treated patients (88% of whom were taking ACE inhibitors), there were too few patients with moderate or severe renal insufficiency to definitely answer the ACE inhibitor–aspririn interaction question.
Tumour necrosis factor (TNF) may have a multifaceted contribution to the progression of the disease in CHF. Blockade of TNF, now an established therapy for rheumatoid arthritis and other autoimmune disorders, has been studied in patients with established CHF. Neither the TNF-receptor fusion protein etanercept or the monoclonal antibody infliximab resulted in beneficial outcomes in this setting.36
COMORBIDITY AND POLYPHARMACY
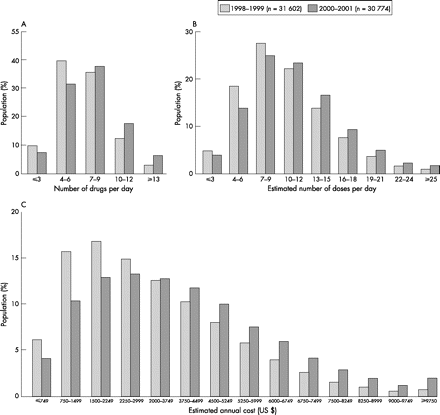
In the patient with CHF with complex comorbidities, physicians typically face the challenge of managing not a single condition but multiple conditions requiring multiple drugs. A recent study of the chronic drugs prescribed at discharge to patients in the US aged ⩾65 years hospitalised for CHF revealed that the mean number of drugs was 6.8, representing 10.1 daily doses (fig 2).37 Unfortunately, little evidence is available to guide this inevitable polypharmacotherapy in patients with CHF and multiple comorbidities. Typically, most CHF trials, either implicitly or explicitly, excluded older patients and patients with multiple comorbidities. In addition, some trials implement run-in periods to assess tolerance to regimens—an approach that may constrain the applicability of the results. Given the lack of data, how should one approach polypharmacy in CHF? Collaborative disease management programmes that include the careful review of drug lists have been shown to reduce hospital admission rates and reduce the costs of care.38 Whenever possible, patients with CHF with multiple competing comorbidities and polypharmacy, need to be enrolled in such programmes. Regardless of the availability of disease management programmes, clinicians need to have systems in place to review medication lists carefully at every visit of a patient, with the goal of eliminating drugs that are not known to provide a clear benefit. When initiating new medications, particular attention needs to be paid to the possibility of adverse drug interactions. In treating coexisting conditions, many commonly used drugs need to be avoided whenever possible in patients with HF, based on known pharmacological principles and recommendations from guidelines.
Distribution of the number of long-term prescription medications (A), estimated number of doses per day (B) and estimated annual cost for long-term medications (C) provided at hospital discharge to elderly patients in the US, hospitalised with heart failure. Reprinted with permission from Masoudi FA, Baillie CA, Wang Y, et al. The complexity and cost of drug regimens of older patients hospitalized with heart failure in the United States, 1998–2001. Arch Intern Med 2005;165:2069–76.37 Copyright 2005 American Medical Association.
CONCLUSIONS
In conclusion, non-cardiac comorbidity frequently complicates CHF care particularly in elderly patients with CHF. Indeed, these are the patients most likely encountered in clinical practice. Under-representation of patients with comorbidities in large CHF clinical trials makes generalisation of trial findings to these patients somewhat difficult. Clinical research must adapt to ensure its relevance, and trials need to include not just young patients with systolic dysfunction and little comorbidity. Ongoing studies enrolling the often ignored group of elderly patients with preserved systolic function, such as the Perindopril in Elderly People-CHF study, are an encouraging development but only represent the beginning of a necessary trend. Future trials must also focus on optimal strategies for the comprehensive management of the patient with CHF with multiple comorbidities rather than the isolated effects of single drugs in younger patients with few or no comorbidities.
REFERENCES
Supplementary materials
Files in this Data Supplement:
Footnotes
-
Published Online First 17 February 2006
-
Competing interests: None.