Article Text
Abstract
Background Many studies have shown that cardiac sympathetic nerve activity evaluated by [123I]m-iodobenzylguanidine ([123I]MIBG) scintigraphic study during a stable period is useful for determining the prognosis of patients with chronic heart failure.
Objective To examine whether results of this imaging method performed 3 weeks after the onset of ST-segment elevation myocardial infarction (STEMI) are a reliable prognostic marker for patients with STEMI.
Methods The study analysed findings for 213 consecutive patients with STEMI undergoing [123I]MIBG scintigraphy. The left ventricular (LV) end-diastolic and end-systolic volume and LV ejection fraction (EF) were determined by left ventriculography or echocardiography 3 weeks after the onset of STEMI. The delayed total defect score, heart-to-mediastinum ratio and washout rate (WR) were also determined from [123I]MIBG scintigraphy at the same time.
Results Of the 213 patients, 46 experienced major adverse cardiac events (MACE) during the study. The median follow-up period was 982 days. Patients were divided into an event-free group (n=167; 78.4%) and a MACE group (n=46; 21.6%). The LV and [123I]MIBG scintigraphic parameters in the event-free group were better than those in the MACE group. Multivariate Cox regression analyses revealed that WR was a significant predictor of MACE along with oral nicorandil (ATP-sensitive potassium channel opener) treatment and undergoing percutaneous coronary intervention. On Kaplan–Meier analysis, the event-free rate of patients with a WR<40% was significantly higher than that in patients with a WR≥40% (p<0.001). Even when confined to patients with LVEF>45%, WR was a predictor of MACE, pump failure death, cardiac death and progression of heart failure in patients with STEMI.
Conclusion WR evaluated by [123I]MIBG scintigraphy 3 weeks after the onset of STEMI is a significant predictor of MACE in patients with STEMI, independent of LVEF.
- Myocardial infarction
- prognosis
- sympathetic nervous system
- scintigraphy
- STEMI
Statistics from Altmetric.com
Activation of the sympathetic nervous system is one of the cardinal pathophysiological abnormalities associated with failing of the human heart.1 Plasma norepinephrine concentrations correspondingly affect the morbidity and mortality of patients with acute myocardial infarction (MI).2 Myocardial imaging with [123I]m-iodobenzylguanidine ([123I]MIBG), an analogue of norepinephrine, is useful for detecting abnormalities in the myocardial adrenergic nervous system in patients with chronic heart failure (CHF).3 4 Furthermore, cardiac sympathetic nerve activity evaluated by [123I]MIBG scintigraphy has useful prognostic value in patients with CHF.4–6 However, there have been no reports on the prognostic value of [123I]MIBG scintigraphy in patients with acute MI, especially ST-segment elevation MI (STEMI). In this study, we examined whether results of [123I]MIBG scintigraphic study performed 3 weeks after the onset of STEMI are a reliable prognostic marker by long-term monitoring of patients with STEMI. Moreover, we sought to evaluate whether this imaging method has prognostic value independent of left ventricular (LV) parameters in these patients.
Patients and methods
Study patients
From December 2000 through April 2008, 412 patients admitted to our institution for STEMI were considered the study population. The diagnosis of STEMI was made on the basis of chest pain >30 min in duration, ST-segment elevation >2 mm in two electrocardiographic (ECG) leads and more than threefold increase in serum creatine kinase activity. In the acute phase, all patients were treated in a standard fashion, including percutaneous coronary intervention (PCI). Patients were excluded from the study if they had primary hepatic failure, severe renal failure or active cancer (38 patients). Moreover, patients with severe heart failure requiring mechanical support (mechanical ventilation, intra-aortic balloon pumping, LV assist device or cardiac resynchronisation therapy) and those requiring heart transplantation were also excluded (68 patients) (figure 1).
Flow diagram of participants in this study. LVG, left ventriculography; MIBG, m-iodobenzylguanidine scintigraphy; TTE, transthoracic echocardiography.
In the stable period, all patients were treated with oral drugs for MI, including antiplatelet treatments, ACE inhibitors, angiotensin receptor blockers, β blockers, nitrates and nicorandil (ATP-sensitive potassium channel opener). Patients treated with tricyclic antidepressant drugs or other serotonin reuptake inhibitors were excluded (12 patients). This study was approved by the ethics review board of our institution, and informed written consent was obtained from all patients except 15, who were thus excluded (figure 1).
Study protocol
We performed [123I]MIBG scintigraphy 3 weeks after the onset of STEMI; this date was considered day 0 of observation. However, 35 patients were excluded from this study because scintigraphy had not been performed. (Eight of 35 patients had not undergone [123I]MIBG scintigraphy owing to cardiac or non-cardiac death other than death due to heart failure.) Moreover, contrast left ventriculography or echocardiography was performed at the same time as [123I]MIBG imaging, but 16 patients were excluded because LV measurements had not been obtained. Medical management of the patients was directed by an internist or cardiologist from our institution, and [123I]MIBG scintigraphic parameters were available to them. However, 15 patients were excluded because they were lost to follow-up after undergoing [123I]MIBG scintigraphy. In this study, 1 December 2008 was considered the end of follow-up.
Finally, we were able to follow-up 213 patients with highly reliable information on their prognosis obtained from the patients themselves, their families or their affiliated hospitals (figure 1). The 213 study patients comprised 158 men and 55 women with a mean age of 68.1 years (range 44–90).
In this study, major adverse cardiac events (MACE) were cardiac death and hospitalisation because of heart failure. Cardiac deaths were classified as sudden death, pump failure death or death secondary to MI. Death was considered sudden if it was unexpected, based on the patient's clinical status, and if it occurred out of the hospital within 15 min of the onset of unexpected symptoms or during sleep. Death during hospitalisation for worsening of congestive symptoms was considered pump failure death.
[123I]MIBG imaging
The method of [123I]MIBG imaging has been described previously.7 The [123I]MIBG was obtained from a commercial source (FUJIFILM RI Pharma Co Ltd, Tokyo, Japan). Patients were injected intravenously with [123I]MIBG (111 MBq) while in supine position. At 15 min and 4 h after injection, static data were acquired in the anterior view with a single-head gamma camera (Millennium MPR, GE Medical Systems, Waukesha, Wisconsin, USA) equipped with a low-energy, general-purpose, parallel-hole collimator. Static images on a 128×128 matrix were collected for 5 min with a 20% window centred on 159 keV, corresponding to the 123I photopeak. After the static planar images were acquired, single photon emission computed tomographic (SPECT) images were obtained. The camera was rotated over 180° from the 45° right anterior oblique position to the 45° left posterior oblique position in 32 views with an acquisition time of 40 s per view. Scans were acquired in a 64×64 matrix, and the images were reconstructed by a filtered back-projection method.
Global analysis of [123I]MIBG imaging
The heart/mediastinum count (H/M) ratio was determined from anterior planar delayed [123I]MIBG images using the method reported by Merlet et al.4 The washout rate (WR) was calculated as {([H]−[M])early−([H]−[M])delayed}/([H]−[M])early × 100 (%), where [H] = mean count/pixel in the left ventricle and [M] = mean count/pixel in the upper mediastinum. In this study, time decay was not corrected for in the calculation of WR.
The delayed myocardial SPECT images of each patient were divided into the 17 segments recommended by the American Heart Association. Regional tracer uptake was assessed semiquantitatively using a five-point scoring system (0=normal uptake; 1=mildly reduced uptake; 2=moderately reduced uptake; 3=significantly reduced uptake; 4=no uptake). Total defect score (TDS) was calculated as the sum of all defect scores.
Regional analysis of [123I]MIBG imaging
To evaluate regional adrenergic dysfunction in patients with STEMI on SPECT images, we calculated a regional defect score (RDS) for each of the 17 segments. Then the infarcted RDS index (RDSI) was calculated as the average RDS of the culprit segments. The non-infarcted RDSI was also calculated as the average RDS of the non-culprit segments.
Left ventricular parameters
The LV end-diastolic volume (EDV), LV end-systolic volume (ESV) and LV ejection fraction (LVEF) were determined by contrast left ventriculography or echocardiography 3 weeks after the onset of STEMI (at the same time as [123I]MIBG imaging). In our institution, follow-up catheterisation (coronary angiography and left ventriculography) is routinely performed after 3 weeks in patients with STEMI undergoing PCI. These LV parameters were calculated by the area–length method in blind fashion by an independent observer, as previously reported.7 For patients not undergoing left ventriculography, echocardiography was performed after 3 weeks. The echocardiographic measurements were calculated using the modified method of Simpson.8
In our institution, the correlations of left ventriculographic and echocardiographic LV measurements were high (EDV; r=0.97, ESV; r=0.96, LVEF; r=0.95, all p<0.001, as evaluated by linear regression analysis). We therefore treated these measurements similarly.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 for Windows (SPSS Inc). Numerical results are expressed as the mean (SD). In all analyses, p<0.05 was considered significant. Patients were divided into an event-free group and a MACE group (table 1). Comparisons of baseline categorical data between the two groups were performed by two-sided χ2 tests, while differences between continuous variables were evaluated using the unpaired t test and Mann–Whitney U test.
Clinical characteristics of the patients who had and who did not have MACE
A Cox proportional hazards regression analysis was performed to identify independent predictors of MACE using variables including clinical characteristics, risk factors, each pharmacotherapeutic agent and undergoing PCI. We evaluated three models for prediction of MACE. In addition to clinical characteristics, risk factors, and treatments, one model included LV parameters as variables (model A), the other included MIBG scintigraphic parameters (model B), while the last model included LV and MIBG scintigraphic parameters (model C) (table 2). The forward stepwise method was used for the multivariate analyses, with entry and removal p values set at 0.05. Moreover, we compared the effectiveness of three models according to the value of the Akaike information criterion.9 For patients with both preserved and reduced LVEF, pump failure death, cardiac death and progression of heart failure (ie, hospitalisation because of heart failure) were the other end points, and evaluation was performed using univariate and adjusted Cox proportional hazards model analyses (table 3). The event date was the date of MACE, and the censoring date was that of the end of follow-up or change in drug after undergoing [123I]MIBG scintigraphy.
Multivariate predictors of major adverse cardiac events
Washout rate for prediction of MACE, pump failure death, cardiac death and progression of HF
The cut-off values of [123I]MIBG scintigraphic or LV parameters for predicting MACE-free survival were identified by receiver operating characteristic (ROC) analysis. Kaplan–Meier survival curves were used for survival comparisons between patient groups stratified according to these cut-off points, and compared by the log-rank test.
Results
Follow-up periods and prognosis of patients
The median follow-up period was 982 days (14–2856 days) for all study patients, and MACE occurred in 46 of the 213 patients (21.6%). MACE in this study occurred a median of 462 days (31–2246 days) after [123I]MIBG scintigraphy. Sudden death accounted for five cases (11%), pump failure death accounted for 13 cases (28%) and the remaining 28 cases were due to hospitalisation because of heart failure (61%). No patients died secondary to MI. Patients were divided into an event-free group (n=167) and a MACE group (n=46) (table 1).
Comparison of clinical characteristics, left ventricular and [123I]MIBG scintigraphic parameters, and pharmacotherapy between the event-free and MACE groups
The clinical characteristics and LV and scintigraphic parameters of the study patients are shown in table 1. Gender, incidence of anterior MI, smoking, obesity, history of hypertension, diabetes mellitus and dyslipidaemia were similar in the two groups. Age in the event-free group was significantly lower than that in the MACE group. The rate of undergoing PCI was significantly higher and peak serum creatine kinase concentration significantly lower in the event-free group than in the MACE group. With respect to pharmacotherapy, the rates of use of β blockers and nicorandil in the event-free group were significantly higher than those in the MACE group.
Of left ventricular parameters, EDV and ESV were significantly lower in the event-free group than in the MACE group. Furthermore, LVEF was significantly higher in the event-free group than in the MACE group. Of [123I]MIBG scintigraphic parameters, TDS and WR were significantly lower in the event-free group than in the MACE group. The H/M ratio was significantly higher in the event-free group than in the MACE group. The infarcted RDSI and non-infarcted RDSI were significantly lower in the event-free group than in the MACE group.
Multivariate predictors of major adverse cardiac events
Table 2 shows the results of multivariate Cox proportional hazards model analyses of MACE. In the multivariate analysis with model A (including clinical characteristics, risk factors and treatments, and LV parameters as variables), LVEF was a significant predictor of MACE, along with nicorandil treatment and undergoing PCI. In model B (including MIBG scintigraphic parameters as variables), WR was a significant predictor of MACE, along with nicorandil treatment and undergoing PCI. Finally, in model C (including LV and MIBG scintigraphic parameters as variables), WR was the best predictor of MACE, along with nicorandil treatment and undergoing PCI. Model B was better than model A and model C as determined by Akaike information criterion value.
Kaplan–Meier survival analysis
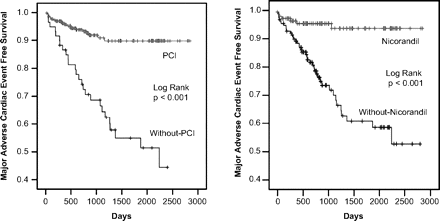
As representative parameters determined by multivariate analysis, we evaluated cut-off values of WR and LVEF for prediction of MACE-free and prepared ROC curves (area under the curve 0.909 and 0.778, respectively). The best cut-off values of WR and LVEF with the highest sensitivity and specificity were 40% and 45%, respectively. Figure 2 shows that the MACE-free survival rates were significantly higher in patients with a WR<40% than in those with a WR≥40% (p<0.001) and in patients with a LVEF>45% than in those with a LVEF≤45% (p=0.001). Figure 3 also shows that MACE-free survival rates were significantly higher in patients undergoing PCI than in those not undergoing it (p<0.001) and in patients with oral nicorandil treatment than in those without it (p<0.001).
Kaplan–Meier survival curves of cumulative MACE-free survival rates in patients with ST-segment elevation myocardial infarction divided into two groups according to WR (left side) and LVEF (right side). Patients with a WR<40% or LVEF>45% had significantly higher MACE-free rates than their counterparts (p<0.001 and p=0.001, respectively). LVEF, left ventricular ejection fraction; MACE, major adverse cardiac events; WR, washout rate.
Kaplan–Meier survival curves of cumulative MACE-free survival rates in patients with ST-segment elevation myocardial infarction divided into two groups according to performance of PCI (left side) and oral nicorandil treatment (right side). Patients undergoing PCI or with oral nicorandil treatment had significantly higher MACE-free rates than without these treatments (all p<0.001). MACE, major adverse cardiac events; PCI, percutaneous coronary intervention.
Washout rate for the prediction of MACE, pump failure death, cardiac death and progression of heart failure in patients with preserved and reduced LVEF
Table 3 shows WR for the prediction of MACE, pump failure death, cardiac death and progression of heart failure in patients with LVEF>45% and ≤45% evaluated by univariate and adjusted Cox proportional hazards model analyses. In patients with LVEF≤45%, WR was a predictor of these cardiac events. Even when confined to patients with LVEF>45%, WR was also a significant predictor of MACE, pump failure death, cardiac death and progression of heart failure.
Discussion
During the follow-up period, 46 patients experienced MACE among our patients with STEMI. WR evaluated by [123I]MIBG scintigraphy 3 weeks after the onset of STEMI was significantly lower in the event-free group (n=167) than in the MACE group (n=46). Cox proportional hazards model analysis showed that WR was the best predictor of MACE. We examined the sensitivity and specificity of various cut-off values of WR for predicting MACE-free survival. We prepared ROC curves, and concluded that the best cut-off value for WR, with the highest sensitivity and specificity, was 40%. MACE-free rate was significantly higher in the patients with WR<40% than in those with a WR≥40%. Even when confined to patients with preserved LVEF, WR was a significant predictor of MACE, pump failure death, cardiac death and progression of heart failure.
[123I]MIBG is an analogue of the adrenergic-neuronal-blocking agent guanethidine, and is thought to use the same myocardial uptake and release mechanisms as norepinephrine.10 Since myocardial norepinephrine concentration and [123I]MIBG uptake are correlated in patients with heart failure,11 cardiac [123I]MIBG imaging may be useful for detecting abnormalities of the myocardial adrenergic nervous system in the failing human heart.3 4 Cardiac sympathetic nerve activity as evaluated by [123I]MIBG scintigraphy also has useful prognostic value in patients with CHF.4–6 The multicentre trial by Jacobson et al5 reported that delayed H/M ratio was a predictor of cardiac events in 237 patients with CHF with reduced cardiac function. Moreover, Tamaki et al6 concluded that WR was a powerful predictor of cardiac sudden death in 106 patients with CHF. However, to our knowledge, there have been no reports on the prognostic value of [123I]MIBG scintigraphy in patients with STEMI. Our findings demonstrate that WR evaluated by [123I]MIBG scintigraphy 3 weeks after the onset of STEMI is most useful for predicting event-free survival.
In this study, we performed [123I]MIBG scintigraphy at 3 weeks after STEMI onset. The timing of performance of this imaging modality is very important, since the changes in pattern of uptake on [123I]MIBG scintigraphy after MI are dynamic.12 The reduction of cardiac [123I]MIBG accumulation at 24 h after onset of MI was relatively mild, and gradually grew more pronounced until 2 weeks after MI. Uptake subsequently tended to recover.12 These findings suggest that testing at 3 weeks after the onset of STEMI is best, since findings of [123I]MIBG scintigraphy are quite stable during this phase, as shown in our previous study.7
Plasma norepinephrine concentrations are increased in the failing human heart, and affect the morbidity and mortality of patients with acute MI.2 However, in daily clinical management, we often experience wide differences between plasma norepinephrine concentration and [123I]MIBG uptake in patients with various heart diseases.13 14 For this reason, plasma norepinephrine concentration has been reported to be influenced by the metabolites of norepinephrine in cardiac myocytes,15 whereas [123I]MIBG uptake is known not to be influenced by them.10 [123I]MIBG imaging may thus reflect the status of cardiac sympathetic nerve activity more accurately than plasma norepinephrine concentration. We therefore speculate that a [123I]MIBG scintigraphic study may be more useful as a prognostic marker in patients with STEMI than plasma norepinephrine concentration, although we did not measure the latter parameter.
It is known that large infarct size promotes progression of heart failure and increases cardiac events in patients with MI.16 Although we did not perform perfusion scintigraphy, regional denervation area evaluated by [123I]MIBG SPECT imaging is known to correlate with infarct or scar size after MI.17 Moreover, Kramer et al18 reported that increased sympathetic denervation in adjacent non-infarcted regions leads to LV remodelling after acute MI. In this study, both infarcted and non-infarcted RDSI in the MACE group were significantly higher than those in the event-free group. However, these parameters were not identified on multivariate Cox proportional hazards regression analysis. We therefore speculate that WR evaluated by [123I]MIBG scintigraphy may be more useful than infarct or scar size for predicting cardiac events in patients with STEMI.
In general, decreased cardiac function has been shown to be an independent predictor of cardiac events in patients with MI.19 In this study, multivariate analysis performed using the previously reported conventional model including LV parameters (model A) identified LVEF as a predictor of MACE, and another model including [123I]MIBG scintigraphic parameters (model B) identified WR as a predictor. Another multivariate analysis using our new original model including both parameters (model C) identified WR, but not LVEF, as a predictor of MACE. Therefore, in this study, WR may have been a better predictor of event-free survival than LVEF in patients with STEMI. Furthermore, our findings demonstrate for the first time that WR could be a predictor of MACE, pump failure death, cardiac death and progression of heart failure in patients with LVEF>45%. Because there have been few established methods for the prediction of cardiac events in patients with STEMI and preserved systolic function, our findings suggesting the potential usefulness of [123I]MIBG scintigraphy even in this population have significant clinical implications. However, which factor is the best predictor of cardiac events in patients with STEMI needs to be determined in larger numbers of patients.
Nicorandil, a drug with both nitrate-like and ATP-sensitive potassium channel-activating properties, has been reported to have cardioprotective effects in ischaemic myocardium.20 21 The Impact Of Nicorandil in Angina (IONA) study, a completed randomised placebo control trial, clearly demonstrated significant reductions in major coronary events following oral nicorandil administration to patients with stable angina.22 Several potential mechanisms have been proposed for nicorandil's cardioprotective effects: (a) reduction of pre- and afterload23; (b) improvement of myocardial perfusion24; (c) pharmacological preconditioning25; (d) prevention of Ca2+ overload by opening of ATP-sensitive potassium channels26 and (e) free radical scavenging and neutrophil-modulating effects.27 We previously reported that adding nicorandil to standard treatment improves cardiac sympathetic nerve activity and prevents LV remodelling in patient with acute MI28 or ischaemic cardiomyopathy with reduced LVEF.29 However, in those studies,28 29 we could not evaluate the prognostic value of long-term administration of nicorandil. In this study, oral nicorandil treatment ameliorated MACE, as determined by multivariate analysis. Thus, based on findings of a previous study22 and our own results, we speculate that oral nicorandil treatment reduces morbidity and mortality in patients with stable angina and also in those with MI.
Conclusion
The WR obtained by [123I]MIBG scintigraphy 3 weeks after the onset of STEMI was significantly lower in the event-free group than in the MACE group. Multivariate Cox proportional hazards model analysis showed that WR was a significant predictor of MACE and had incremental value over LVEF. Furthermore, the event-free rate in patients with low WR was significantly higher than that in patients with high WR. These findings indicate that [123I]MIBG scintigraphy results can be used as independent predictors of MACE in patients with STEMI.
Acknowledgments
The authors thank Kenji Ushida for expert advice on statistics.
References
Footnotes
Linked articles 209007.
See Editorial, p 1
Competing interests None.
Patient consent Obtained.
Ethics approval This study was conducted with the approval of the https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&recptno=R000001675&type=summary&language=E
Provenance and peer review Not commissioned; externally peer reviewed.