Article Text
Abstract
In this review discuss the application of cardiac magnetic resonance (CMR) to the evaluation and quantification of mitral regurgitation and provide a systematic literature review for comparisons with echocardiography. Using the 2015 Preferred Reporting Items for Systematic Reviews and Meta-Analyses methodology, we searched Medline and PubMed for original research articles published since 2000 that provided data on the quantification of mitral regurgitation by CMR. We identified 220 articles of which 33 were included. Four main techniques of mitral regurgitation quantification were identified. Reproducibility varied substantially between papers but was high overall for all techniques. However, quantification differed between the techniques studied. When compared with two-dimensional echocardiography, mitral regurgitation fraction and regurgitant volume measured by CMR were comparable but typically lower. CMR has high reproducibility for the quantification of mitral regurgitation in experienced centres, but further technological refinement is needed. An integrated and standardised approach that combines multiple techniques is recommended for optimal reproducibility and precise mitral regurgitation quantification. Definitive outcome studies using CMR as a basis for treatment are lacking but needed.
Statistics from Altmetric.com
Introduction
The decision for mitral valve surgery for mitral regurgitation (MR) hinges on an integrated assessment of symptoms, left ventricular (LV) size and function, and an accurate non-invasive assessment of the mechanism and severity of MR. Echocardiography is the most commonly used method for the comprehensive evaluation of MR but may be limited by poor acoustic windows, eccentric or multiple jets and geometric assumptions. When performed in experienced centres, CMR has emerged as a complementary method to determine MR severity.
The aim of this review is to discuss CMR techniques used for the evaluation of MR and the reproducibility and correlation of these techniques with echocardiography.
Methods
Eligibility criteria
A systematic review was performed in accordance with the 2015 Preferred Reporting Items for Systematic Reviews and Meta-Analyses methodology.1 PubMed and Medline were queried for studies published since 2000. Search terms of ‘cardiac magnetic resonance’ and ‘mitral regurgitation’ or ‘mitral insufficiency’ identified 220 potential studies for inclusion. Two reviewers selected articles with adjudication performed by consensus. Exclusion criteria were as follows: (1) studies that used qualitative data without evaluating quantitative CMR techniques, (2) review articles or editorials; (3) studies that did not use modern imaging sequences such as steady-state free precession (SSFP) imaging; and (4) studies with fewer than 10 subjects or non-human subjects. Sections on reproducibility included studies that reported intraobserver or interobserver reproducibility. Comparison with echocardiography included studies that either compared CMR with at least one semiquantitative or fully quantitative echocardiography modality, such as proximal isovelocity surface area (PISA) or used an integrated approach recommended by current guidelines.2 Thirty-three studies (15%) met inclusion criteria and were included in this review.
Methods of MR quantification
Several techniques derive similar measurements, and comparison is valuable for internal validation as part of an integrated approach to MR evaluation (table 1). Some have advocated the additional use of direct mitral anatomic regurgitant orifice planimetry but this technique requires further validation prior to routine clinical use.
Studies of quantitation of MR by CMR
LV stroke volume and aortic flow
The most commonly used CMR technique for quantification of MR severity is the comparison of LV stroke volume (LVsv) with anterograde aortic flow (Ao) (LVsv-Ao; figure 1). To determine LVsv, the LV endocardial border is manually contoured in end diastole and end systole from a short-axis SSFP cine stack. Total aortic anterograde flow is measured using integration of phase-contrast (or velocity-encoded) images obtained in the proximal aorta. The calculated MR volume (MRVol) is the difference between LVsv (which includes both MR and antegrade aortic volumes) and antegrade Ao volumes. The regurgitation fraction (RF) is the ratio of MRVol:LVsv.
Calculation of regurgitation volume by subtracting aortic forward flow from left ventricular stroke volume (LVsv). LV endocardial contours are traced in systole and diastole from a short-axis stack from apex to base with special attention to the basal slices. Aortic forward flow is measured using through-plane phase-contrast MRI. MRVol, mitral regurgitation volume; RF, regurgitation fraction.
Advantages
This method is efficiently incorporated into a CMR study, as all the necessary measurements are included in a standard acquisition. Unlike other CMR techniques, the LVsv-Ao method is not affected by the presence of concomitant aortic, pulmonic or tricuspid valve disease.
Potential pitfalls
While phase-contrast measurement of Ao flow is highly reproducible, variations in LVsv measurements can lead to variability in MR quantification. Specifically, irregular heart rates with atrial fibrillation or inability of an adequate breath hold can obfuscate sharp contours of the ventricular borders as well as alter the phase-contrast signals.
In addition, there are two accepted methods for contouring the LV endocardium although there is not universal agreement for the preferred method (figure 2):
Trabeculations and papillary muscles can be included in the myocardial mass by manually tracing around the contours to exclude from the blood pool. While this method may be closer to ‘true’ stroke volume, this technique increases interobserver variability.
Trabeculations and papillary muscles can be included as part of the blood pool and excluded from the myocardial mass, similar to echocardiography. These so-called rounded contours have higher interobserver reproducibility, but this technique results in larger measured LV end-diastolic and LV end-systolic volumes. This increases both LVsv and subsequently the calculated MRVol. Ejection fraction is altered by 2%–7% depending on technique although the absolute effect on MRVol has not been reported.9–11 Unfortunately, the lack of standardised methods for tracing LV contours and centre-to-centre variability exacerbate measurement error.12
The effect of papillary muscles and trabeculations. Two different methods of drawing the left ventricular (LV) endocardial contour. There is no standardisation as to whether the papillary muscles and trabeculations are included in the blood pool or the myocardial mass. If the trabeculations are individually contoured (red solid line), the LV mass will be larger but the LV volumes will be smaller. If the trabeculations are included in the blood pool (yellow dotted line), then the LV volumes will be smaller. The differences are most pronounced in patients with prominent trabeculations such as this patient with Fabry's disease where the mitral regurgitation was moderate (regurgitation volume 19 mL/beat, regurgitation fraction 26%) when trabeculations were included in the blood pool but trivial (4 mL/beat, 6%) when the trabeculations were included in the myocardial mass.
It is also important to account for basal through-plane motion of the mitral annulus for the LVsv-Ao technique. Basal slices can contain both portions of the LV and the left atrium and, cross-reference with long-axis localisers is helpful. One convention is to include a slice in the LV volume if the blood pool is surrounded by >50% myocardium.13 ,14 While this convention allows for reproducibility, misidentification of portions of the left atrium as ventricle leads to overestimation in the MRVol. Using this technique, half of healthy volunteers (without any visible MR) had a calculated MRVol >10 mL/beat.3 To minimise these limitations, we favour the standardisation of techniques within a centre with reporting of the technique used in order to enhance centre-to-centre comparisons.
Comparison of ventricular stroke volumes (LVsv-RVsv)
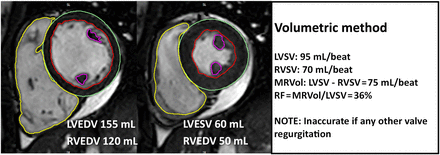
MRVol can also be calculated by comparing the LV and right ventricular stroke volumes (LVsv-RVsv). In the absence of valve disease or shunt lesion, these should be equal and serves as an internal control. In patients with isolated MR, LVsv will be higher than RVsv and the difference is the MRVol (figure 3).
Calculation of regurgitation volume by subtracting right ventricular stroke volume (RVsv) from left ventricular stroke volume (LVsv). RV and LV endocardial contours are traced in systole and diastole. In the absence of any other valvular regurgitation or shunt, the difference should equal the MRVol. MRVol, mitral regurgitation volume; RF, regurgitation fraction.
Advantages
MRVol and RF can be calculated from a single short-axis stack acquired in a standard CMR protocol without the need for phase-contrast velocity-encoded flow images.
Potential pitfalls
Similar to the LVsv-Ao measurements, LV endocardial contour tracings remain important although additional meticulous attention to the tracing of correct RV contours is also critical as the RV tends to have greater variability than LV measurements.14 ,15 ,16 Additionally, the LVsv-RVsv technique is only accurate in patients with isolated MR, as intracardiac shunt lesions or concomitant aortic, tricuspid or pulmonic valve regurgitation will alter LVsv or RVsv.
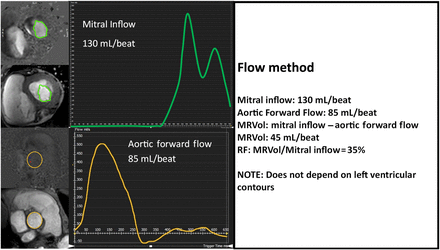
Comparison of mitral inflow with aortic forward flow (mitral annular flow method)
The mitral annular flow method uses a through-plane flow sample placed at the midpoint of the mitral leaflets while they are open in diastole. Contours are traced around the diastolic mitral inflow signal (figure 4) and the systolic component is ignored. Antegrade Ao volumes are calculated using velocity-encoded flow images similar to the other techniques.
Calculation of regurgitation volume by subtracting aortic forward flow from mitral inflow. Through-plane phase-contrast MRI is performed near the mitral leaflet tips in diastole and also in the proximal descending aorta. MRVol is the difference between mitral inflow and aortic forward flow. MRVol, mitral regurgitation volume; RF, regurgitation fraction.
Potential pitfalls
Prescribing optimal through-plane samples in a moving mitral valve annulus is difficult and the resulting inflow measurements may be compromised. Dynamic through-plane motion of the mitral annulus is also common, making accurate flow assessment even more challenging. Automatic border detection of waveforms is rarely effective as the shape of the mitral inflow wave changes during diastole. Therefore, each phase must be contoured individually by hand, adding to processing time and variability.
Direct MR measurement
An infrequently used technique is quantification of MRVol using phase contrast aligned to the plane of the MR jet.6 ,17 ,18 MRVol is directly measured using a high maximal velocity encoding setting to avoid aliasing.
Advantages
While other techniques require at least two separate measurements to calculate MRVol, this technique relies on a single measurement.
Potential pitfalls
In theory, a direct measurement of flows would be ideal as there is no calculation involved in its derivation. While this technique is routinely used for aortic or pulmonic regurgitation, MR measurement is technically difficult to apply due to the dynamic motion of the mitral annulus during ventricular systole. This is made even more challenging in certain valve pathologies, such as eccentric jets or in mitral valve prolapse (MVP), in which the MR jet orientation changes throughout systole and results in underestimation of MRVol.6
Reproducibility of CMR techniques
Studies that reported interobserver or intraobserver reproducibility using quantitative measures of MR severity are detailed in table 1. Most techniques demonstrate high intraobserver and interobserver reproducibility.
Several studies compared the reproducibility of multiple techniques in a single cohort. In a prospective study of 26 subjects with MR in our centre, Cawley et al4 showed that the LVsv-Ao flow technique had better intraobserver and interobserver reproducibility when compared with the LVsv-RVsv technique. Polte et al3 compared MRVol calculations using LVsv-Ao, LVsv-RVsv and mitral annular flow methods. The LVsv-Ao resulted in the highest MRVol of the three techniques, but overestimated MR in healthy volunteers in whom no MR was visibly present by 15–20 mL/beat. Interobserver variability was lowest for the mitral annular inflow method and highest for LVsv-Ao. Myerson et al17 compared the direct MR measurement with other methods in 55 patients and showed that LVsv-Ao had numerically better reproducibility. However, when restricted to the patients with low heart rate variability (80% of the cohort), the reproducibility of the mitral annular inflow method had the best reproducibility.
Uretsky et al5 prospectively compared CMR (using the LVsv-Ao technique) and transthoracic echocardiogram (TTE) (by proximal isovolumic surface area or PISA measures) to predict the response to mitral valve replacement in patients with chronic MR. CMR-derived regurgitant volume (RVol) had better reproducibility than TTE (CMR ICC 0.9 vs TTE ICC 0.65). The authors found only moderate correlation between the two modalities (r=0.4, p<0.0001) and that RVol derived by CMR was significantly lower than RVol derived by TTE. Importantly, the authors found that RVol as determined by CMR (but not TTE) was associated with postoperative remodelling following mitral valve replacement suggesting that CMR was more reproducible and may be more accurate than TTE in determining MR severity or that TTE overestimated MR severity in many patients.
To date, no single technique has emerged as the most reproducible method for CMR quantification of MR. Operators should be aware of the advantages and limitations of each technique and how technical considerations such as the treatment of trabeculations or through-plane flow measurement affect MR quantification. We recommend standardising techniques and integrating the information gained from multiple CMR MR measurements—as is performed in echocardiography—to optimise reproducibility and precision of MR measures.
Prognostic data
Long-term prognostic data for patients with degenerative (primary) and functional (ischaemic or secondary) MR are available to assess risk of progression to surgery or to development of adverse outcomes of heart failure, hospital admissions or death.19 ,20 However, little prognostic data exist to predict outcomes on the basis of CMR alone. CMR, however, provides superior volumetric data for the left and right ventricle (table 2) as well as an assessment of myocardial viability with late gadolinium enhancement (LGE). In theory, this could better predict patients at risk of adverse events although definitive data are lacking. In patients with regional wall motion and ischaemic (secondary) MR, this may be of assistance in planning revascularisation options. Gadolinium-enhanced magnetic resonance angiography is also useful in assessing the aorta and peripheral access vessels for planning of percutaneous mitral valve repair.
Comparison of echocardiography and CMR in evaluating cardiac structure and function
Special populations
In this section, we describe situations in which the CMR ‘toolkit’ of different pulse sequences and tissue characterisation can provide specific and unique and additive value to the assessment of mitral valve disease in a variety of different populations.
Mitral valve prolapse
CMR is capable of delineating the anatomy of mitral leaflet pathology with excellent correlation with echocardiography.21 In patients with MVP, CMR can accurately differentiate flail and prolapsing leaflet scallops. This nuanced information does however require a carefully planned set of SSFP cine images through the mitral valve leaflets to correctly assess leaflet segments and the commissures. In MVP, CMR also identifies other metrics important for determining aetiology of MR and surgical planning, such as anterior mitral leaflet length, leaflet displacement, leaflet thickness and presence of flail segments.22 CMR can also be used to measure annular dimensions, identify annular splaying and provide accurate assessment of LV volumes to plan intervention.
Percutaneous mitral valve repair
Percutaneous mitral valve repair using a transcatheter clip system (MitraClip) is a newer technology to treat severe MR in patients at high surgical risk. Echocardiographic evaluation of residual MR following percutaneous mitral valve repair is challenging due to acoustic shadowing from of the clips, eccentric MR jets, multiple regurgitant jets with dynamic flow patterns and altered valve anatomy due to the edge-to-edge repair. CMR quantitation of residual regurgitation after MitraClip has higher reproducibility than to Doppler echocardiography.6 There is no published literature about the use of CMR to evaluate other emerging technologies, such as percutaneous mitral valve replacements or annular cinching devices, but is an important topic for future study.
Hypertrophic cardiomyopathy
CMR is often used in the diagnosis of hypertrophic cardiomyopathy due to its ability to assess wall thickness, particularly in apical and concentric varieties that are more challenging for echocardiography. In addition, CMR can provide incremental information compared with echocardiography through the ability to identify and quantify myocardial fibrosis using LGE imaging and T1 mapping. HCM is often associated with dynamic MR due to systolic anterior motion of the mitral valve, leading to a late-systolic posteriorly directed MR. MR is present at rest in one-third of patients and inducible with stress in another one-third of patients.23 CMR can be used to accurately identify the position and number of papillary muscles that, when apically displaced, contribute to the mechanism of outflow tract obstruction. Abnormal chordal attachment to the mitral valve and abnormal mitral valve leaflet length well seen by TTE can also be seen by CMR although often the chordal apparatus can be difficult to evaluate due to rapid movement.24
Of note, spectral Doppler echocardiography is superior to CMR for the evaluation of dynamic LV outflow tract (LVOT) gradients. CMR can evaluate flow with velocity-encoded imaging, although the long breath holds and acquisition time are not ideal for dynamically changing LVOT gradients. In addition, thick acquisition slices tend to underestimate peak velocities via signal averaging.22 Thus, CMR and echocardiography can be considered complementary in the assessment of patients with HCM and coexisting MR (table 2).
Ischaemic MR
The differentiation of ischaemic MR from other mitral valve pathologies is of importance as the management centres around revascularisation rather than a primary surgical or interventional repair. The true identification of ischaemic MR is difficult and often relies on evidence of physiological aberrations in coronary perfusion. In theory, CMR may inform the presence of ischaemic MR by identifying scar via LGE imaging and/or ischaemia using resting and stress myocardial perfusion.
Comparison of MR by echocardiography with CMR
Review of the literature reveals a wide range of methodologies used for the comparison between echocardiography and CMR. In general, studies tended to be small and limited by the lack of a definitive gold standard for comparison. The most common grading scheme for MR uses an integrative approach endorsed by major society guidelines.25 Quantification of MRVol most commonly uses the PISA method, and occasionally the Doppler volumetric method. Many other techniques are available including semiquantitative approaches, such as comparing the colour Doppler area with the left atrial area as well as more quantitative 3D PISA, 2D PISA integrated throughout systole, M-mode PISA, real-time 3D full volume colour Doppler and the 3D stroke volume method. However, the significant heterogeneity in the MR literature complicated simple comparisons between echocardiography and CMR. There is also little consistency in whether transthoracic echocardiography, transoesophageal echocardiography or both were compared with CMR. Recognising this heterogeneity, we primarily accepted literature that evaluated native valve disease using the most commonly used quantitative techniques for both echocardiography and CMR. Table 3 summarises these selected studies that directly compare quantitative echocardiography with CMR.
Comparison of quantitation of mitral regurgitation by echocardiography and CMR
The most common CMR technique compared with echocardiography is the LVsv-Ao technique, although some authors use the LVsv-RVsv and mitral inflow methods. In many (but not all) of the published literature, CMR appears to systematically underestimate RVol compared with echocardiography, particularly when compared with PISA (table 3). This highlights the importance of understanding the nuances of local practice patterns when applying these data to clinical care and the need for further systematic research.
Summary
In experienced centres, CMR is a highly reproducible technique for quantitative assessment of MR which benefits from the use of multiple, complimentary measurements that provide internal controls and validation. In addition to basic quantification, CMR provides tools for a detailed analysis of myocardial anatomy and tissue characterisation, which can supplement the clinical management. Our review found that despite its excellent reproducibility there was a subtle variation in the subtleties of CMR image acquisition and processing which likely explained our variable results in comparison with echocardiography. As with any imaging modality, training and expertise matters, and the integration of CMR into clinical practice is best performed by an experienced centre dedicated to valvular heart disease. We also advocate that a good understanding of the benefits of CMR requires a dialogue and understanding of the local practice patterns in regard to the subtleties of image acquisition and standardisation. Although the current data are promising, additional data from larger trials are needed to validate clinical management decisions and prognosis based on CMR measurements. As faster acquisition techniques become available and are integrated with investigational sequences, such as 4D flow and ultrahigh-field (7 Tesla) imaging, the future of CMR in the evaluation of valvular heart disease is constantly evolving. In experienced hands, CMR remains a useful and complementary technique to echocardiography for the assessment of MR.
References
Footnotes
Contributors EVK: drafting of manuscript, creation of figures, critical review and final approval. JL: drafting of manuscript and final approval. KRB and CH-C: drafting of manuscript, critical review and final approval.
Competing interests None declared.
Provenance and peer review Commissioned; externally peer reviewed.