Article Text
Abstract
Background The relative safety of percutaneous left ventricular assist device (pVAD) and intra-aortic balloon pump (IABP) in patients with cardiogenic shock after acute myocardial infarction remain unknown.
Methods Multiple databases were searched to identify articles comparing pVAD and IABP. An unadjusted OR was used to calculate hard clinical outcomes and mortality differences on a random effect model.
Results Seven studies comprising 26 726 patients (1110 in the pVAD group and 25 616 in the IABP group) were included. The odds of all-cause mortality (OR 0.57, 95% CI 0.47 to 0.68, p=<0.00001) and need for revascularisation (OR 0.16, 95% CI, 0.07 to 0.38, p=<0.0001) were significantly reduced in patients receiving pVAD compared with IABP. The odds of stroke (OR 1.12, 95% CI 0.14 to 9.17, p=0.91), acute limb ischaemia (OR=2.48, 95% CI 0.39 to 15.66, p=0.33) and major bleeding (OR 0.36, 95% CI 0.01 to 25.39, p=0.64) were not significantly different between the two groups. A sensitivity analysis based on the exclusion of the study with the largest weight showed no difference in the mortality difference between the two mechanical circulatory support devices.
Conclusions In patients with acute myocardial infarction complicated by cardiogenic shock, there is no significant difference in the adjusted risk of all-cause mortality, major bleeding, stroke and limb ischaemia between the devices. Randomised trials are warranted to investigate further the safety and efficacy of these devices in patients with cardiogenic shock.
- cardiogenic shock
- myocardial infarction
- meta-analysis
Data availability statement
Data are available upon reasonable request. Data available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key questions
What is already known about this subject?
Coronary Mechanical circulatory support devices have recently been advanced in design to be used in the acute setting of cardiogenic shock (CS).
There is a lack of sufficient data surrounding the relative safety of percutaneous left ventricular assist device (pVAD) and intra-aortic balloon pump (IABP) in patients with CS after acute myocardial infarction.
What does this study add?
Our study demonstrated that odds of all-cause mortality and the need for revascularisation were significantly reduced in patients receiving pVAD compared with IABP. However, there was no significant difference in the adjusted risk of all-cause mortality, major bleeding, stroke and limb ischaemia between the devices.
How might this impact on clinical practice?
pVAD is comparable to IABP in terms of cardiovascular outcomes, and choice of selection depends on interventionists and the clinical presentation.
Randomised trials are warranted to investigate further the safety and efficacy of these devices in patients with CS.
Introduction
About 81% of cardiogenic shock (CS) events occur as a complication of acute myocardial infarction (AMI), resulting in end-organ damage and tissue hypoperfusion.1–3 While inotropes are still widely administered, their use is associated with deterring limitations ranging from increased cardiac oxygen consumption and arrhythmias to inadequate circulatory support.4 Mechanical circulatory support (MCS) devices originally designed to improve cardiac output in patients with end-stage heart failure and have recently been advanced in design to be used in the acute setting.5 it has been proposed that MCS improves ventricular function compared with inotropes.
The choice of appropriate MCS, however, has long been debated. Contemporary studies have shown a steep increase in the utilisation of percutaneous left ventricular assist device (pVAD) compared with intra-aortic balloon pump (IABP), but data on the relative preference of one device over the other are scarce and conflicting.6–8 A large retrospective study by Khera et al reported a higher unadjusted in-hospital mortality of 38.3% in patients with CS and pVAD use.8 This number dropped significantly to only 8.4% in patients without CS, which leaves a large window for questions given the heterogeneity and complexity of CS.8 These findings contrast the findings of a randomised control trial (RCT) by Thiele et al, which reported a 30-day mortality of 69.7% in no intervention group compared with a 38.8% mortality with IABP and the IMPRESS trial, which showed a 50% 30-day mortality rate in 24 patients with IABP.1 6 Due to the insufficient conflicting data on the use of different MCS, we sought to perform a meta-analysis on the relative merits of pVAD and IABP.
Methods
The PubMed, Embase and Cochrane databases were queried with various combinations of medical subject headings (MeSH) to identify relevant articles. There were no language or time restrictions placed. Backward snowballing was performed to retrieve unidentified studies that were missed on the initial search. The keywords ‘pVAD’, ‘Impella’ and ‘intra-aortic balloon pump’ were combined with a list of MeSH terms for cardiogenic shock and acute myocardial infarction using the Boolean operators. Independent authors screened results from all possible combinations. All RCTs until July 2020, comparing the safety of pVAD versus IABP in CS, were evaluated for inclusion. Data were extracted into a standard excel sheet and were evaluated before analysis. The primary endpoint was all-cause mortality; the secondary outcome included revascularisation, acute limb ischaemia, stroke and major bleeding events after the procedure.
The statistical analysis was performed using the random-effects model to calculate an unadjusted OR for dichotomous variables. The probability value of p<0.05 was considered statistically significant. The ‘test for overall effect’ was reported as z value corroborating the inference from the 95% CI. Higgins I-squared (I2) statistic model was used to assess variations in outcomes of the included studies. I2 values of 50% or less corresponded to low to moderate and 75% or higher indicated large amounts of heterogeneity. A sensitivity analysis censored by the weight of study and sample size was also computed to nullify the effect of potentially influential studies. Publication bias was illustrated graphically using a funnel plot. The quality assessment of the included articles was performed using the Cochrane risk of bias-2 tool, where each study was screened for five different types of bias (selection, performance, detection, attrition and reporting bias). All statistical analyses were performed using the Cochrane Review Manager (RevMan) V.5.4.
Results
Search results and study characteristics
The initial search revealed 1302 articles. After removing irrelevant (613) and duplicate items (505), 184 studies were selected for full-text review. Of these, 167 articles were excluded based on our selection criteria, 7 articles (4 RCTs and 3 retrospective studies) qualified for quantitative analysis. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram is shown in figure 1.
PRISMA flow diagram of the included studies. RCT, randomised control trial; PRISMA, Preferred ReportingItems for Systematic Reviews and Meta-Analyses.
Due to the novelty of these devices, we expected paucity in the number of RCTs comparing one against the other; therefore, our study was designed to include non-randomised observational retrospective studies as well. A total of 26 726 patients, 1110 in the pVAD group and 25 616 in the IABP group, were included. The mean age of patients in the Impella group was 65 years and for the IABP group, 63 years, comprising 65% and 68% male patients, respectively. Baseline characteristics of the pVAD and IABP groups were comparable, including diabetes mellitus (27% vs 36%), hypertension (53% vs 68%), hyperlipidaemia (31% vs 27%), history of myocardial infarction (34% vs 23%) and smoking (55% vs 40%), respectively. The PROTECT II clinical trial used pVAD V.2.5 circulatory support system and IABP in acute coronary syndrome patients. These patients had New York Heart Association class of III–IV and a mean SYNTAX score of 58.9 and 54.9 in the pVAD and IABP groups, respectively. The IMPELLA-STIC randomised study and the retrospective study by Schwartz used pVAD LP5.0 pump and IABP in their study population.9 The (Impella LP2.5 vs IABP in Cardiogenic SHOCK) ISAR-SHOCK trial studied the haemodynamic effects of pVAD and IABP only in patients with CS. The IMPRESS trial included patients who were mechanically ventilated. Khera et al used a National Inpatient Sample data to examine the use of pVAD on the same day as percutaneous intervention (PCI) in the study population. The prospective observational study by Pieri et al compared pVAD 2.5 with IABP in their study population. The mean follow-up was 3.4 months. The detailed baseline characteristics are given in table 1.
Baseline characteristics of the included studies
Pooled analysis of overall studies
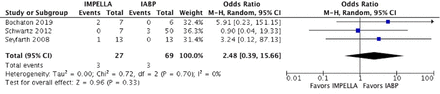
At a mean follow-up of 3.4 months, a significantly lower rate of all-cause mortality (OR 0.57, 95% CI 0.47 to 0.68, p<0.00001) and the need for repeat revascularisation (OR 0.16, 95% CI 0.07 to 0.38, p<0.0001) was observed in patients receiving pVAD post AMI–CS compared with IABP (figures 2 and 3). The incidence of stroke (OR 1.12, 95% CI 0.14 to 9.17, p=0.91), acute limb ischaemia (OR=2.48, 95% CI 0.39 to 15.66, p=0.33) and major bleeding events (OR=0.36, 95% CI 0.01 to 25.39, p=0.64) were statistically nonsignificant between the two groups (figures 4–6). There was no heterogeneity among the adverse outcomes of all the included studies (I2=0%), with the exception of studies comparing the major bleeding events (I2=82%).
Forest plot for mortality showing an individual and pooled OR for studies comparing pVAD to IABP in patients with acute coronary syndrome-induced cardiogenic shock. pVAD, percutaneous left ventricular assist device;IABP, intra-aortic balloon pump.
Forest plot for repeat revascularisation showing an individual and pooled OR for studies comparing pVAD to IABP in patients with ACS-induced cardiogenic shock. pVAD, percutaneous left ventricular assist device;IABP, intra-aortic balloon pump.
Forest plot for stroke showing an individual and pooled OR for studies comparing pVAD to IABP in patients with ACS-induced cardiogenic shock. pVAD, percutaneous left ventricular assist device;IABP, intra-aortic balloon pump.
Forest plot for major bleeding showing an individual and pooled OR for studies comparing pVAD to IABP in patients with ACS induced cardiogenic shock. pVAD, percutaneous left ventricular assist device;IABP, intra-aortic balloon pump.
Forest plot for acute limb ischaemia showing an individual and pooled OR for studies comparing pVAD to IABP in patients with ACS induced cardiogenic shock. pVAD, percutaneous left ventricular assist device;IABP, intra-aortic balloon pump.
Sensitivity analysis
In contrast to the pooled results, a sensitivity analysis based on the exclusion of Khera et al study showed an identical rate of all-cause mortality between the two devices (OR 0.96, 95% CI 0.42 to 2.16, p=0.62) (figure 7).
Forest plot for sensitivity analysis of mortality (Khera et al excluded) showing an individual and pooled OR for studies comparing pVAD to IABP in patients with ACS induced cardiogenic shock. pVAD, percutaneous left ventricular assist device;IABP, intra-aortic balloon pump.
Publication bias
Our funnel plot was symmetrical on visual assessment, indicating that the limited scatter was due to sampling variation and not due to publication bias. The plot’s vertical axis used SE to estimate the sample size of the study, plotting large population studies on top and smaller at the bottom. The horizontal spread reflected the power and effect size of the included studies (figure 8).
Funnel plot showing a lower risk of publication bias across studies. (A) Mortality, (B) limb ischaemia, (C) bleeding, (D) cerebrovascular accident, (E) revascularisation.
Discussion
To our knowledge, this is the first meta-analysis to exclusively compare the safety of percutaneous ventricular assist device (pVAD) against IABP in patients having CS after AMI. Our results showed a significantly lower rate of mortality in the pVAD group; however, these results were attenuated on a sensitivity analysis, indicating that the differential mortality rate was driven by one retrospective study of Khera et al. While IABP was numerically favoured due to a 12% and 60% lower risk of stroke and acute limb ischaemia and pVAD was superior due to a 64% lower rate of major bleeding, none of these differences reach a level of statistical significance. Although the need for revascularisation was significantly lower in the pVAD group, this outcome’s biological plausibility warrants a large-scale study.
Postprocedure peripheral vascular ischaemia is a well-known haemodynamic complication of MCS. A report by the University of Michigan showed a 14% higher vascular repair rate and a 2% amputation rate following IABP.10 Similarly, an observational study on 90 patients by Abaunza et al reported 12% and 4% rates of acute limb ischaemia and limb loss, respectively.11 In theory, IABP should have superior protection against acute limb ischaemia due to its mechanism of action by increasing diastolic pressure and afterload reduction. On the contrary, pVAD acts by reducing ventricular workload and reducing myocardial oxygen consumption and stroke work. However, our results did not show a significant difference in stroke incidence or acute limb ischaemia between the two groups.
Previous studies suggested that the use of pVAD is associated with a 2–4 times higher rate of major bleeding than IABP.1 6 12–14 The IMPELLA-STIC randomised study went further to question the safety of Impella in terms of bleeding.15 According to Bochaton et al, major bleeding was observed in five out of seven patients in the Impella +IABP group and none in the IABP group. Our findings contrast the results of these previous studies by demonstrating no significant difference in the two devices in major bleeding events. This could be explained by the rapid advances in the pVAD technology and interventionist skills.11 Some studies suggest that pVAD protects the need for repeated PCI in high-risk patients.16–18 The impella 2.5 arm of the PROTECT II trial showed 52% lower repeat revascularisation rates at 90 days. Our results are consistent with these findings, showing 47% lower odds of revascularisation in patients receiving pVAD compared with IABP.
It is, however, imperative to interpret our results with caution in light of its limitations due to the inclusion of nonrandomised small-scale data and the impact of unmeasured confounding factors on our findings. Due to the lack of large-scale RCTs, our comparison arms suffered from an imbalance in the group’s clinical characteristics, which prevented excluding residual confounders even after adjustment. Similarly, we could not account for the varying devices used and differing inclusion criteria of the included studies. Of the included trials, the IMPRESS trial included patients with a mean age of 58 years, significantly younger than the population included by Seyfarth et al, where the mean age was 66 years. About 92% of the patients in the former trial had cardiac arrest before randomisation, and CS was defined as SBP <90, in contrast to the later study where CS definition was based on mean arterial pressures.6 12
Similarly, the study by Schwartz et al used a different approach; due to training, simplicity and rapidity, IABP was the first-choice device, especially in emergent cases, while pVAD was only deployed after failed trials of IABP and vasopressor support. Consequently, 70% and 16% of patients in the IABP group had STEMI and cardiac arrest, respectively. Patients in the pVAD group were more severely compromised and had a 1.3 mean vasopressor score before treatment initiation.9 Pieri et al were a prospective observational study aimed at investigating a multidevice approach where pVAD was used on top of another haemodynamic support device (either VA ECMO or IABP).19 Additionally, Bochtan et al compared pVAD, which generated an output of 5.0 L/min compared with other studies using impellas with a maximum output of 2.5 L/min. Collectively, these limitations call for the need for a large-scale RCT to determine the relative merits of these devices.
In conclusion, in patients with AMI complicated by CS, there is no significant difference in the adjusted risk of all-cause mortality, major bleeding, stroke and limb ischaemia between the devices. Randomised trials are warranted to investigate further the safety and efficacy of these devices in patients with CS.
Data availability statement
Data are available upon reasonable request. Data available upon reasonable request.
Ethics statements
References
Footnotes
Twitter @chadialraies
Contributors WU and MZ contributed equally to this manuscript as first co-authors. Conceptualisation: WU, MZ, MCA. Methodology: WU, MM, YS, MZ. Formal analysis: WU. Original draft writing: WU, AB, GA, SZ, MZ. Journal formatting: MZ. Critical review and editing: MZ, HMP, DG, MCA.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.