Abstract
Aims: Coronary artery disease (CAD) is frequently present in patients with severe aortic stenosis (AS) undergoing transcatheter aortic valve implantation (TAVI). While revascularisation affects peri-operative outcome in patients undergoing surgical aortic valve replacement, the impact of percutaneous coronary intervention (PCI) in patients undergoing TAVI is not well established.
Methods and results: Consecutive patients with severe AS undergoing TAVI were prospectively included into the Bern TAVI registry. In patients with CAD, myocardium at risk was assessed using the DUKE myocardial jeopardy score. Revascularisation was performed by means of PCI either staged or concomitant at the time of TAVI. Among 256 patients undergoing TAVI, 167 patients had CAD and 59 patients underwent either staged (n=23) or concomitant (n=36) PCI. Clinical outcome at 30 days was similar for patients undergoing isolated TAVI as compared with TAVI combined with PCI in terms of death (5.6% versus 10.2%, p=0.24), major stroke (4.1% versus 3.4%, p=1.00), and the VARC combined safety endpoint (31.0% versus 23.7%, p=0.33). A stratified analysis of outcomes according to presence of CAD or revascularisation showed no difference during long-term follow-up (log rank p=0.16).
Conclusions: CAD is frequent among patients with severe AS undergoing TAVI. Among carefully selected patients, revascularisation by means of PCI can be safely performed in addition to TAVI either as a staged or a concomitant intervention.
Introduction
Coronary artery disease is common among elderly patients with degenerative aortic valve stenosis (AS) and an indication to undergo surgical aortic valve replacement (SAVR) or transcatheter aortic valve implantation (TAVI). The ESC guidelines on valvular heart disease recommend to perform complete revascularisation among patients with severe AS undergoing SAVR to improve long-term outcomes1. However, as compared with isolated SAVR, the combination of coronary artery bypass grafting (CABG) with SAVR is associated with increased peri-operative complications and mortality2-4.
Patients undergoing TAVI following interdisciplinary discussion of treatment allocation represent a high-risk patient population, and the indication for revascularisation of significant CAD by means of percutaneous coronary intervention (PCI) is under debate. A joint position paper of the European Society of Cardiology (ESC) and the European Association of Cardio-Thoracic Surgery (EACTS) states that severe CAD not amenable for PCI represents a formal contraindication for TAVI5. Previous observational studies reporting outcomes of patients undergoing TAVI have observed a prevalence of CAD in the range of 52-68%6-11. An analysis from two feasibility studies reported a substantial increase in periprocedural mortality among patients undergoing TAVI with CAD suggesting a prognostic relevance of CAD irrespective of revascularisation status12. In current clinical practice, it is recommended to postpone TAVI for one month after PCI in order to minimise the risk of the TAVI procedure. Until recently the safety of TAVI has therefore just been investigated isolated from concomitant revascularisation procedures. Only one small study has evaluated the safety and feasibility of concomitant or staged PCI in patients undergoing TAVI so far, and reported a 30-day mortality rate of 7.1%13. The purpose of the present study was therefore to assess the prevalence and impact of CAD as well as the safety and feasibility of revascularisation by means of PCI on clinical outcomes among high-risk patients with severe AS undergoing TAVI.
Methods
Patient population
Patients with severe symptomatic AS referred to a tertiary care facility considered at increased risk for SAVR were enrolled in a prospective registry initiated in July 2007. Octogenarians were eligible for inclusion in the presence of a logistic EuroSCORE >15%, and patients <80 years of age qualified for inclusion if at least one of the following conditions was present: previous cardiac surgery, chronic obstructive pulmonary disease (forced expiratory volume during one second <1.0), severe pulmonary hypertension (>60 mmHg), porcelain aorta, history of radiation therapy to the mediastinum, or BMI <18 kg/m2. Patients with severe aortic regurgitation were excluded. All patients underwent comprehensive evaluation for TAVI using right and left heart catheterisation, CT angiography of the chest and the access site, echocardiography and subspecialty consultations in case of pertinent comorbidities. Assignment to a transcatheter strategy was based on an interdisciplinary consensus by the heart team consisting of interventional cardiologists and cardiac surgeons. The algorithm for treatment allocation and device selection has been reported previously14. The Bern TAVI registry was approved by the local ethics committee and all subjects gave written, informed consent.
Procedures
TAVI was performed using both CE approved devices, the Medtronic CoreValve system (Medtronic, Minneapolis, MN, USA) and the Edwards SAPIEN valve (Edwards Lifesciences, Irvine, CA, USA) through a transfemoral, transapical or trans-subclavian approach according to instructions for use and as previously described. Device and access site selection were driven by anatomical and technical features14. Patients undergoing isolated TAVI were loaded with clopidogrel 300-600 mg the day prior to intervention. PCI for CAD was performed using standard techniques. Before or at the time of the procedure, patients were treated with at least 100 mg of acetylsalicylic acid, a 600 mg loading dose of clopidogrel, and unfractionated heparin 70-100 U/kg. PCI was performed either in a planned intervention prior to TAVI (staged PCI) or at the time of TAVI (concomitant). In case of concomitant PCI, patients first underwent PCI followed by TAVI in the same session.
Data collection
Adverse events were assessed in-hospital, and regular clinical follow-up was performed at 1, 6, and 12 months by means of a clinical visit or a standardised telephone interview. In addition, all patients were contacted within two months of data freezing (October 4, 2010 through November 29, 2010). Municipal civil registries and hospital records were consulted to ascertain vital status. For patients with a suspected event, relevant medical records, discharge letters, and documentation of hospitalisation were systematically collected from treating hospitals and physicians in private practice. All suspected events were adjudicated by an unblinded clinical event committee consisting of cardiac surgeons and interventional cardiologists. Baseline clinical and procedural characteristics and all follow-up data were entered into a dedicated database, held at an academic clinical trials unit (CTU Bern, Bern University Hospital, Switzerland) responsible for central data audits and maintenance of the database.
Definitions
CAD was defined as a stenosis of >50% in at least one coronary artery as assessed during coronary angiography, or a status post previous PCI or CABG. The DUKE myocardial jeopardy score15 was used to assess myocardium at risk. In brief, all three coronary arteries were divided in a total of six segments assigned two points each, resulting in a maximum score of 12. The SYNTAX score16 was used to assess the complexity of CAD in patients undergoing concomitant or staged PCI. All endpoints were defined according to the Valve Academic Research Consortium (VARC) criteria17.
Cardiovascular death involved any death due to a proximate cardiac cause or death of unknown cause, as well as all procedure-related deaths and death caused by non-coronary vascular conditions such as cerebrovascular disease, pulmonary embolism, or other vascular disease. Periprocedural myocardial infarction was considered in case of new ischaemic symptoms or signs in the presence of elevated cardiac biomarkers (two or more post-procedure samples that were >6-8 hours apart with a 20% increase in the second sample and a peak value exceeding 10x the 99th percentile upper reference limit (URL), or a peak value exceeding 5x the 99th percentile URL with new pathological Q-waves in at least two contiguous leads) within 72 hours after the index procedure. Major stroke was defined as a rapid onset of focal or global neurological deficit of ≥24 hours duration requiring therapeutic intervention, or documentation of a new intracranial defect using MRI or CT-scan. Transient ischaemic attack (TIA) was considered in case of a neurologic deficit with complete regression within 24 hours of onset. Bleeding complications were classified as life-threatening or disabling (1) in case of bleeding into a critical area or organ, or (2) bleeding causing hypovolemic shock or requiring vasopressors or surgery, or (3) with an overt source of bleeding with a decrease in haemoglobin ≥5 g/dl or packed red blood cells transfusion ≥4 units. Major bleeding encompassed overt bleeding associated with a decrease in haemoglobin level ≥3.0 g/dl. Major vascular access site complications were defined as access-related vascular injuries leading to either death, need for blood transfusions (≥4 units), percutaneous or surgical intervention, or irreversible end-organ damage. Minor vascular complications included failure of percutaneous access site closure resulting in interventional or surgical correction. For the definition of kidney injury the modified RIFLE classification (Risk, Injury, Failure, Low output, End-stage kidney disease) was used which was based upon changes in serum creatinine within 72 hours after the procedure. Stage 1 was defined as an increase of serum creatinine to 150-200% (or an increase of ≥26.4 µmol/l), stage 2 was determined as an increase of baseline creatinine to 200-300%, and stage 3 was considered in case of an increase in creatinine of ≥300% with an acute increase of at least 44 µmol/l.
Statistical analysis
All statistical analyses were performed using SPSS Statistics version 17.0 (SPSS Inc.,Chicago, IL, USA). Continuous variables are presented as mean ±standard deviation (SD) and were compared by means of a two-sided students T-test. Categorical data are expressed as frequency (percentages), and were compared using the chi-square and Fishers exact tests. Survival was estimated using the Kaplan Meier method. A p value <0.05 was considered statistically significant.
Results
Among 452 patients enrolled into the prospective Bern TAVI registry between July 2007 and September 2010, 257 patients were assigned to TAVI using a transfemoral, transapical or trans-subclavian approach (Figure 1). CAD was found in 167 (65%) patients, of whom 59 (35%) patients underwent either staged (n=23) or concomitant (n=36) PCI in addition to TAVI. Of the remaining 108 patients with CAD but no revascularisation procedure, 53 patients (49%) had been completely revascularised prior to TAVI, whereas 55 patients (51%) had an incomplete revascularisation status (DUKE myocardial jeopardy score ≥1). Among patients with CAD, the decision to perform staged or concomitant revascularisation by means of PCI was justified by a significantly higher DUKE myocardial jeopardy score (5.0±3.2 versus 2.1±2.7, p=0.03) reflecting the amount of ischaemic myocardium. Chronic total occlusions and distal segments or side branches with a small area at risk were left untreated.
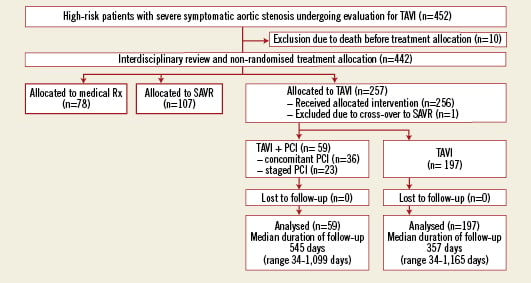
Figure 1. Patient flow according to the CONSORT statement.
The prevalence of angina was similar among patients with CAD undergoing revascularisation as compared to those with CAD not undergoing revascularisation (29% versus 32%, p=0.86). Moreover, there was no difference in the prevalence of angina between patients with or without CAD (31% versus 27%, p=0.57). Staged PCI was performed 34±26 days prior to TAVI. Concomitant PCI was planned in all but one case, in which partial occlusion of the left main occurred after transapical valve implantation leading to an emergent PCI. Besides concomitant revascularisation, three patients underwent concomitant structural heart interventions. In addition to TAVI, one patient underwent closure of a persistent foramen ovale, one patient underwent PCI and occlusion of the left atrial appendage (LAA), and one patient underwent PCI as well as closure of an atrial septal defect (ASD) and LAA-closure. Patients undergoing staged or concomitant PCI were older (83.6±4.8 years versus 81.7±6.5 years, p=0.04), had more frequently a history of prior PCI (17.3% versus 40.7%, p<0.001), and a higher estimated interventional risk as assessed by the STS score (7.6±6.2 versus 6.1±4.5, p=0.03) (Table1).
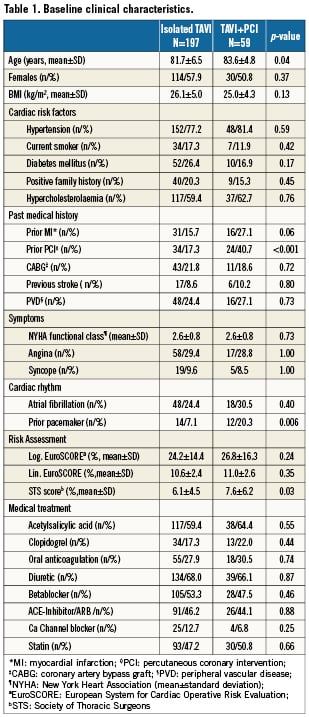
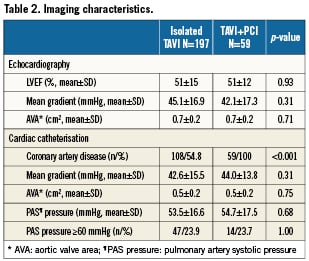
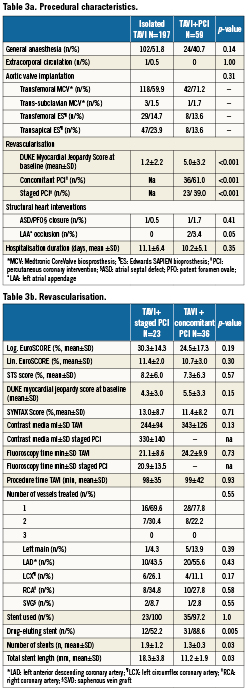
There were no differences between patients with or without revascularisation in terms of left ventricular function, mean transvalvular aortic gradient or pulmonary hypertension (Table 2). Fifty-nine (23%) patients underwent either a transapical or left trans-subclavian approach accounting for the rather high rate of general anaesthesia (Table 3a). Procedural success of PCI (residual stenosis <20%) was noted in 93% of patients. In four patients, the stenosed lesion could not be passed with a coronary guidewire and the procedure was therefore unsuccessful. DUKE myocardial jeopardy scores after PCI amounted to 1.8±2.4 and 1.1±1.8 for patients with concomitant and staged interventions, respectively. Those with a staged approach were treated with a higher number of stents (1.9±1.2 versus 1.3±0.3, p=0.03), accompanied by a longer total stent length (18.3±3.8 mm versus 11.2±1.9 mm, p=0.03). In contrast, patients with concomitant PCI more frequently received drug-eluting stents (88.6% versus 52.2%; p=0.005). There was no significant difference with regard to the amount of contrast used or fluoroscopy time in patients undergoing staged or concomitant PCI, respectively (Table 3b).
The VARC combined safety endpoint occurred with similar frequency among patients with or without CAD irrespective of revascularisation status (28% versus 32%, p=0.67). There was also no difference in terms of the individual components of the VARC combined safety endpoint through 30 days between patients with or without revascularisation (Table 4). A separate analysis comparing short-term clinical outcome among patients without revascularisation and those undergoing staged or concomitant PCI revealed no significant differences between the three groups with regard to the VARC combined safety endpoint (Figure 2). Likewise, after exclusion of patients undergoing staged PCI a direct comparison between patients undergoing TAVI with concomitant PCI and those undergoing isolated TAVI showed no significant differences with respect to overall mortality (11.1% versus 5.6%, p=0.26), major stroke (5.6% versus 4.1%, p=0.66), and the VARC combined safety endpoint (22.2% versus 31.0%, p=0.33). Clinical outcomes up to two years after the intervention did not show any difference in terms of overall mortality (Figure 3). None of the three patients with concomitant structural interventions experienced a serious adverse event at the time of intervention or at follow-up.
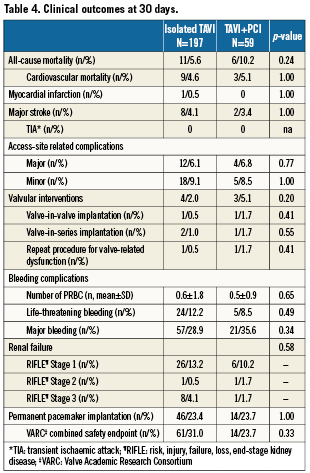
Figure 2. Clinical outcome at 30 days according to the Valve Academic Research Consortium (VARC) safety endpoint for patients undergoing TAVI only, TAVI with staged PCI, and TAVI with concomitant PCI.
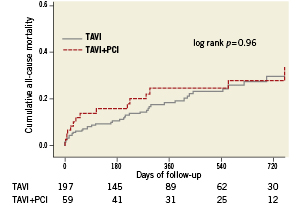
Figure 3. Kaplan-Meier survival analysis up to two years of follow-up for patients undergoing TAVI only, and patients undergoing staged or concomitant revascularisation.
Patients undergoing isolated TAVI were analysed with regard to CAD and revascularisation status. Among patients undergoing isolated TAVI (n=197), 108 patients (55%) had CAD, of whom 55 patients (51%) were incompletely revascularised (DUKE myocardial jeopardy score ≥1). Patients with complete or incomplete revascularisation of CAD were younger as compared to patients with no CAD (80.9±6.4 versus 80.2±7.1 versus 83.1±6.0 years, p=0.02), were more frequently men (28/52.8% versus 33/60.0% versus 22/24.7%, p<0.001), had a higher prevalence of diabetes (19/35.8% versus 19/34.5% versus 14/15.7%, p=0.009) and dyslipidaemia (41/77.4% versus 39/70.9% versus 37/41.6%, p<0.001), and had a higher risk as assessed by the logistic EuroSCORE (23.1±11.2% versus 30.2±18.2% versus 21.1±12.4%, p=0.001). There was no significant difference with regard to mid-term survival among patients undergoing isolated TAVI as a function of completely and incompletely revascularised CAD (Figure 4).
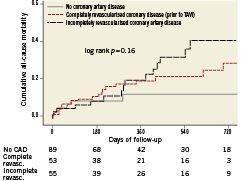
Figure 4. Kaplan-Meier survival analysis up to two years of follow-up for patients undergoing isolated TAVI without CAD, completely revascularised CAD prior to TAVI, or incompletely revascularised CAD.
Discussion
In patients with severe AS, TAVI improves survival as compared to medical treatment in candidates not suitable for SAVR11. Co-existing CAD is observed in more than half of patients qualifying for TAVI6-11 and may require revascularisation in order to alleviate symptoms and improve survival. The key findings of our study are as follows: (1) CAD is common in patients with severe aortic stenosis at increased risk for SAVR; (2) Staged or concomitant PCI is feasible and safe in selected patients with severe AS undergoing TAVI; (3) Complete or incomplete revascularisation of CAD does not appear to adversely impact on mid-term survival.
Our data demonstrate that staged or concomitant PCI in the setting of TAVI is feasible and safe in selected patients with severe AS with similar 30-day mortality for patients undergoing isolated TAVI as compared to those undergoing TAVI combined with PCI. Moreover, other periprocedural complications such as stroke, bleeding and vascular complications occurred with comparable frequencies. These findings contrast with previous reports in the surgical literature suggesting an increased peri-operative risk of the combination of revascularisation by means of CABG and SAVR2-4. In view of the observational nature of the data of the present study, the results should not be interpreted outside the context of clinical decision making on an individual basis within the interdisciplinary heart team. The similar outcome irrespective of revascularisation procedure in the present study may be explained at least in part by the meticulous patient selection within the heart team. Thus, patients with severe multivessel disease have been considered preferentially as candidates for CABG combined with SAVR, whereas patients with proximal lesions, easily accessible for PCI were favoured to undergo staged or concomitant PCI combined with TAVI. A concomitant approach bears the advantage of one single arterial access for the treatment of CAD and AS and might therefore reduce the risk of vascular access complications and bleeding events. In our patient cohort a trend towards a lower incidence of vascular complications and life-threatening bleedings was observed for the approach combining PCI and TAVI in one session (concomitant) and supports this hypothesis. On the other hand, one may argue that the risk of myocardial infarction, stroke and renal failure is increased for patients undergoing concomitant PCI during TAVI, as the procedure time is prolonged and the amount of contrast is larger. In conclusion, if selected appropriately, comparable clinical outcomes with a staged or concomitant PCI strategy can be achieved among patients with severe AS undergoing TAVI. This is consistent with previous findings by Conradi et al reporting a 30-day mortality of 7.1% among 28 patients treated with staged or concomitant PCI13.
CAD was encountered in 65% of patients undergoing TAVI in our cohort, which is consistent with previous observational studies6-10 and the recent PARTNER trial (Placement of AoRtic TraNscathetER Valve Trial)11. CAD was associated with a tenfold increased mortality risk within 30 days after TAVI in a dedicated analysis of two feasibility studies12. In contrast to these findings, we did not observe differences during clinical follow-up between patients with or without CAD irrespective of revascularisation status. Two reasons may account for this discrepancy between our findings and the previous report. First, in the cohort with CAD reported by Dewey et al, patients also had a higher logistic EuroSCORE, lower ejection fraction, and relevant mitral regurgitation which may all have an important influence on clinical outcomes. Second, the extent of CAD might not be comparable between the two populations. Furthermore, completeness of revascularisation was not reported by Dewey but might impact on clinical outcome. In our study population this question cannot be addressed adequately as the proportion of patients incompletely revascularised is relatively small and the extent of myocardium at risk is limited as reflected in the low DUKE myocardial jeopardy score. None of the patients with relevant proximal stenoses were left un-revascularised. The Kaplan-Meier curve yet suggests a potential impact of incomplete revascularisation during long-term follow-up. However this finding must be interpreted with caution in light of the thorough evaluation and selection of patients. An adverse impact of coronary artery disease on clinical outcome is likely to emerge only during extended follow-up. Revascularisation is an upcoming challenge in TAVI and needs to be addressed in larger randomised trials.
The present study has several limitations. First, allocation to revascularisation followed the recommendation of the heart team consensus and was not randomised. Second, the treatment strategy –staged versus concomitant PCI– was left to the discretion of the operator. Both decisions are open to selection bias and may therefore influence outcomes. Lastly, the observational design of this registry including only a limited number of patients must be interpreted with caution.
Conclusion
Coronary artery disease in selected patients with severe aortic stenosis undergoing TAVI can be treated safely by means of PCI, either during a staged procedure or concomitantly during TAVI. The impact of complete coronary revascularisation on clinical outcomes among patients undergoing TAVI remains to be determined.
Conflict of interest statement
Drs. Wenaweser and Windecker receive lecture and consultant fees from Edwards Lifesciences and Medtronic CoreValve. Drs. Huber, Kadner and Carrel receive lecture and consultant fees from Edwards Lifesciences. Dr Buellesfeld is proctor for Medtronic CoreValve, Dr Khattab is proctor for Medtronic CoreValve and Edwards Lifesciences. Dr Meier receives research grants and is a member of the speakers bureau of Johnson and Johnson, Boston Scientific, Medtronic, Abbott, Biotronik, and Biosensors. The other authors have no conflict of interest to declare.




