Abstract
Background: The electrocardiographic QT interval is used to identify drugs with potential harmful effects on cardiac repolarization in drug trials, but the variability of the measurement can mask drug-induced ECG changes. The use of complementary electrocardiographic indices of abnormal repolarization is therefore warranted. Most drugs associated with risk are inhibitors of the rapidly activating delayed rectifier potassium current (Ikr). This current is also inhibited in the congenital type 2 form of the long QT syndrome (LQT2). It is therefore possible that electrocardiographic LQT2 patterns might be used to identify abnormal repolarization patterns induced by drugs.
Objective: To develop distinct T-wave morphology parameters typical of LQT2 and investigate their use as a composite measure for identification of d,l-sotalol (sotalol)-induced changes in T-wave morphology.
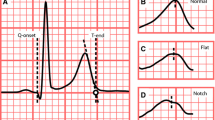
Methods: Three independent study groups were included: a group of 917 healthy subjects and a group of 30 LQT2 carriers were used for the development of T-wave morphology measures. The computerized measure for T-wave morphology (morphology combination score, MCS) was based on asymmetry, flatness and notching, which are typical ECG patterns in LQT2. Blinded to labels, the new morphology measures were tested in a third group of 39 healthy subjects receiving sotalol. Over 3 days the sotalol group received 0, 160 and 320 mg doses, respectively, and a 12-lead Holter ECG was recorded for 22.5 hours each day. Drug-induced prolongation of the heart rate corrected QT interval (QTcF) was compared with changes in the computerized measure for T-wave morphology. Effect sizes for QTcF and MCS were calculated at the time of maximum plasma concentrations and for maximum change from baseline. Accuracy for separating baseline from sotalol recordings was evaluated by area under the receiver operating characteristic curves (AUCs) using all recordings from the time immediately post-dose to maximum change.
Results: MCS separated baseline recordings from sotalol treatment with higher accuracy than QTcF for the 160 mg dose: (AUC) 84% versus 72% and for the 320 mg dose: (AUC) 94% versus 87%, p < 0.001. At maximum serum-plasma concentrations and at maximum individual change from baseline, the effect sizes for QTcF were less than half the effect sizes for MCS, p< 0.001. Effect sizes at peak changes of the mean were up to 3-fold higher for MCS compared with QTcF, p< 0.001. In subjects receiving sotalol, T-wave morphology reached similarity to LQT2, whereas QTcF did not.
Conclusion: Distinct ECG patterns in LQT2 carriers effectively quantified repolarization changes induced by sotalol. Further studies are needed to validate whether this measure has general validity for the identification of drug-induced disturbed repolarization.







Similar content being viewed by others
References
Haverkamp W, Breithart G, Camm AJ, et al. The potential for QT prolongation and proarrhythmia by nonantiarrhythmic drugs: clinical and regulatory implications. Eur Heart J 2000; 21: 1216–30
Sanguinetti MC, Curran ME, Spector PS, et al. Spectrum of HERG K+-channel dysfunction in an inherited cardiac arrhythmia. Proc Natl Acad Sci USA 1996; 93: 2208–12
Lasser KE, Allen PD, Woolhandler SJ, et al. Timing of new black box warnings and withdrawals for prescription medications. JAMA 2002; 287: 2215–20
Roden DM. Drug induced prolongation of the QT interval. N Engl J Med 2004; 350: 1013–22
Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell 2001; 104: 569–80
Sanguinetti MC, Jiang C, Curran ME, et al. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell 1995; 81: 299–7
Vincent GM, Timothy KW, Leppert M, et al. The spectrum of symptoms and QT intervals in carriers of the gene for long-QT syndrome. N Engl J Med 1992; 327: 846–52
Zhang L, Timothy KW, Vincent GM, et al. Spectrum of ST-T wave patterns and repolarization parameters in congenital long QT syndrome: ECG findings identify genotypes. Circulation 2000; 102: 2849–55
Kanters JK, Fanoe S, Larsen LA, et al. T-wave morphology analysis distinguishes between KvLQT1 and HERG mutations in long QT syndrome. Heart Rhythm 2004; 1: 285–92
Lehmann MH, Suzuki F, Fromm BS, et al. T wave ‘humps’ as a potential electrocardiographic marker of the long-QT syndrome. J Am Coll Cardiol 1994; 24: 746–54
Malfatto G, Beria G, Sala S, et al. Quantitative analysis of T wave abnormalities and their prognostic implications in the idiopathic long-QT syndrome. J Am Coll Cardiol 1994; 23: 296–1
Lupoglazoff JM, Denjoy I, Berthet M, et al. Notched T waves on Holter recordings enhance detection of patients with LQT2 (HERG) mutations. Circulation 2001; 103: 1095–1
Merri M, Benhorin J, Alberti M, et al. Electrocardiographic quantitation of ventricular repolarization. Circulation 1989; 80: 1301–8
Benhorin J, Merri M, Alberti M, et al. Long QT syndrome: new electrocardiographic characteristics. Circulation 1990; 82: 521–7
Struijk JJ, Kanters JK, Andersen MP, et al. Classification of the long QT syndrome based on discriminant analysis of T-wave morphology. Med Biol Eng Comput 2006; 44: 543–9
Gima K, Rudy Y. Ionic current basis of electrocardiographic waveforms: a model study. Circ Res 2002; 90: 889–96
Shah RR, Hondeghem LM. Refining detection of drug-induced proarrhythmia: QT interval and TRIaD. Heart Rhythm 2005; 2: 758–72
Antzelevitch C. Heterogeneity of cellular repolarization in LQTS: the role of M cells. Eur Heart J Suppl 2001; 3 Suppl. K: K2–16
Couderc JP, McNitt S, Xia J, et al. Repolarization morphology in adult LQT2 carriers with borderline prolonged QTc interval. Heart Rhythm 2006; 3: 1460–6
Shah RR. Drug-induced QT dispersion: does it predict the risk of Torsade de pointes? J Electrocardol 2005; 38: 10–8
Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT Syndrome. Circulation 1998; 98: 1928–36
Topilski I, Rogowski O, Rosso R, et al. The morphology of the QT interval predicts Torsade de Pointes during acquired bradyarrhythmias. J Am Coll Cardiol 2007; 49: 320–8
Cruz Filho FE, Maia IG, Fagundes ML, et al. Electrical behavior of T-wave polarity alternans in patients with congenital long QT syndrome. J Am Coll Cardiol 2000; 36: 167–73
Thomsen MB, Verduyn SC, Stengl M, et al. Increased short-term variability of repolarization predicts d-sotalol-induced Torsades de Pointes in dogs. Circulation 2004; 110: 2453–9
Hinterseer M, Thomsen MB, Beckmann B-M, et al. Beat-to-beat variability of QT intervals is increased in patients with drug-induced long-QT syndrome: a case control pilot study. Eur Heart J 2008; 29: 185–90
Jensen BT, Abildstrom SZ, Larroude CE, et al. QT dynamics in risk stratification after myocardial infarction. Heart Rhythm 2005; 2: 357–64
Couderc JP, Zareba W, Moss AJ, et al. Identification of sotalol-induced changes in repolarization with T wave area-based repolarization duration parameters. J Electrocardiol 2003; 36: 115–20
Guidance for industry: E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. 2005 [online]. Available from URL: http://www.fda.gov/cber/gdlns/iche14qtc.htm [Accessed 2008 Apr 20]
Sarapa N, Morganroth J, Couderc JP, et al. Electrocardiographic identification of drug-induced QT prolongation: assessment by different recording methods. Ann Non-invasive Electrocardiol 2004; 9: 48–57
Priori SG, Mortara DW, Napolitano C, et al. Evaluation of the spatial aspects of T-wave complexity in the long-QT syndrome. Circulation 1997; 96: 3006–12
Andersen MP, Xue J, Graff C, et al. New descriptors of T-wave morphology are independent of heart rate. J Electrocardiol 2008; 41: 557–61
Andersen MP, Xue JQ, Graff C, et al. A robust method for quantification of ikr-related t-wave morphology abnormalities. Comp Cardiol 2007; 34: 341–4
Glass GV. Integrating findings: the meta-analysis of research. Rev Res Educ 1977; 5: 351–79
Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characcteristic curves derived from the same cases. Radiology 1983; 148: 839–43
Kay GN, Plumb VJ, Arciniegras JG, et al. Torsade de pointes: the long-short initiating sequence and other clinical features: observations in 32 patients. J Am Coll Cardiol 1983; 2: 806–17
Bauman JL, Bauernfeind RA, Hoff JV, et al. Torsade de pointes due to quinidine: observations in 31 patients. Am Heart J 1984; 107: 425–30
Hondeghem LM. Frequency dependence of class I and class III antiarrhythmic agents explaining their antiarrhythmic and proarrhythmic properties. In: Franz MR, editor. Monophasic action potentials: bridging cell and bedside. 1st ed. Armonk: Futura Publishing, 2000: 381–94
Benardeau A, Weissenburger J, Hondeghem L, et al. Effects of the T-type Ca++ channel blocker mibefradil on repolarization of guinea pig, rabbit, dog, monkey, and human cardiac tissue. J Phamacol Exp Ther 2000; 292: 561–75
Sicouri S, Moro S, Litovsky S, et al. Chronic amiodarone reduces transmural dispersion of repolarization in the canine heart. J Cardiovasc Electrophysiol 1997; 8: 1269–79
Smetena P, Pueyo E, Hnatkova K, et al. Effect of amiodarone on the descending limb of the T wave. Am J Cardiol 2003; 92: 742–6
Kanters JK, Haarmark C, Vedel-Larsen E, et al. TpeakTend in long QT syndrome. J Electrocardiol 2008; 41: 603–8
Yamaguchi M, Shimizu M, Ino H, et al. T wave peak-to-end interval and QT dispersion in acquired long QT syndrome: a new index for arrhythmogenicity. Clin Sci 2003; 105: 671–6
Extramiana F, Antzelevitch C. Amplified transmural dispersion of repolarization as the basis for arrhythmogenesis in a canine ventricular-wedge model of the short-QT syndrome. Circulation 2004; 110: 3661–6
Whitley E, Ball J. Statistics review 4: sample size calculations. Crit Care 2002; 6: 335–41
Acknowledgements
No external sources of funding were used to support the conduct of this study. Claus Graff, Mads Andersen, Thomas Hardahl, Jørgen Kanters, Egon Toft and Johannes Struijk are authors of two filed patents describing the T-wave morphology method. A license agreement exists between Aalborg University and GE Healthcare regarding this method. Joel Xue is an employee of GE Healthcare and owns GE Healthcare stock. Michael Christiansen and Henrik Jensen have no financial or personal conflicts of interests to report. The authors thank Pfizer Inc. for providing the data from the sotalol study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Graff, C., Andersen, M.P., Xue, J.Q. et al. Identifying Drug-Induced Repolarization Abnormalities from Distinct ECG Patterns in Congenital Long QT Syndrome. Drug-Safety 32, 599–611 (2009). https://doi.org/10.2165/00002018-200932070-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200932070-00006