-
PDF
- Split View
-
Views
-
Cite
Cite
Holger Thiele, Peter Sick, Enno Boudriot, Klaus-Werner Diederich, Rainer Hambrecht, Josef Niebauer, Gerhard Schuler, Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock, European Heart Journal, Volume 26, Issue 13, July 2005, Pages 1276–1283, https://doi.org/10.1093/eurheartj/ehi161
Close - Share Icon Share
Abstract
Aims Mortality in cardiogenic shock (CS) following acute myocardial infarction (AMI) remains unacceptably high despite percutaneous coronary intervention (PCI) of the infarcted artery and use of intra-aortic balloon pump (IABP) counterpulsation. A newly developed percutaneous left ventricular assist device (VAD) (Tandem Heart™, Cardiac Assist, Pittsburgh, PA, USA) with active circulatory support might have positive haemodynamic effects and decrease mortality.
Methods and results Patients in CS after AMI, with intended PCI of the infarcted artery, were randomized to either IABP (n=20) or percutaneous VAD support (n=21). The primary outcome measure cardiac power index, as well as other haemodynamic and metabolic variables, could be improved more effectively by VAD support from 0.22 [interquartile range (IQR) 0.19–0.30] to 0.37 W/m2 (IQR 0.30–0.47, P<0.001) when compared with IABP from 0.22 (IQR 0.18–0.30) to 0.28 W/m2 (IQR 0.24–0.36, P=0.02; P=0.004 for intergroup comparison). However, complications like severe bleeding (n=19 vs. n=8, P=0.002) or limb ischaemia (n=7 vs. n=0, P=0.009) were encountered more frequently after VAD support, whereas 30 day mortality was similar (IABP 45% vs. VAD 43%, log-rank, P=0.86).
Conclusion Haemodynamic and metabolic parameters can be reversed more effectively by VAD than by standard treatment with IABP. However, more complications were encountered by the highly invasive procedure and by the extracorporeal support.
Introduction
Despite aggressive treatment modalities, such as percutaneous coronary intervention (PCI) and use of intra-aortic balloon pumping (IABP), mortality of cardiogenic shock (CS) complicating acute myocardial infarction (AMI) remains at an unacceptable high level with mortality rates as high as 60–70%.1–3 During the recovery time of myocardial performance following revascularization of the infarcted vessel many patients succumb to low cardiac output. Therefore, an IABP, which can result in initial haemodynamic stabilization,4,5 is the method of choice for mechanical assistance in these patients.6 However, the main limitation of IABP is the lack of active cardiac support and the requirement of a certain residual level of left ventricular function. In many patients with severe depression of left ventricular function, haemodynamic support derived from IABP lacks efficiency to reverse CS.2
In a recent feasibility trial using a percutaneous left ventricular assist device (VAD) with active circulatory support, there were substantial haemodynamic improvements by the VAD and CS mortality was very low.7 This randomized study was designed to assess the haemodynamic effects of IABP in comparison to VAD (Tandem Heart™, Cardiac Assist, Pittsburgh, PA, USA) and to evaluate mortality in patients with CS complicating AMI.
Methods
From August 2000 to December 2003, patients with CS were randomized by drawing sealed envelopes either to IABP (n=20) or to VAD (n=21). Inclusion criteria were the presence of CS complicating AMI and the intention to revascularize the infarcted artery by PCI as first line treatment option. In case of unsuitable coronary anatomy, i.e. left main stenosis, emergency coronary artery bypass grafting was permitted. Operators were urged to limit PCI to the infarcted vessel. However, staged multivessel PCI was possible. Glycoprotein IIb/IIIa receptor antagonists were recommended for TIMI flow less than III or evidence of intracoronary thrombus after PCI. CS was defined as (i) persistent systolic blood pressure <90 mmHg or vasopressors required to maintain blood pressure >90 mmHg; (ii) evidence of endorgan failure (e.g. urine output <30 mL/h, cold skin and extremities, and serum lactate >2 mmol/L); (iii) evidence of elevated left ventricular filling pressures [pulmonary congestion or pulmonary capillary wedge pressure (PCWP) >15 mmHg]; and (iv) cardiac index (CI) <2.1 L/min/m2. Exclusion criteria were age >75 years, mechanical complications of AMI, duration of CS >12 h, right heart failure, sepsis, significant aortic regurgitation, severe cerebral damage, resuscitation >30 min, severe peripheral vascular disease, and other diseases with reduced life expectancy.
For group comparison at baseline, a 30 day mortality predictive score was used.8 The score encompasses variables as age, height, baseline heart rate, baseline systolic blood pressure, time-to-reperfusion, and miscellaneous factors. For patients with prior thrombolytic therapy, the time-to-reperfusion was defined as time-to-thrombolysis and for those with primary PCI as time-to-balloon-inflation.
Patients gave written informed consent, if their mental status permitted; otherwise, the procedure was discussed with their relatives. The protocol was approved by the local Ethics Committee.
Description of the assist systems and implantation procedure
The tandem heart VAD and percutaneous implantation procedure have been described in detail elsewhere.7 In brief, after trans-septal puncture a venous inflow cannula is inserted into the left atrium. Oxygenated blood is drawn from there and returned via a centrifugal pump and via an arterial cannula in the femoral artery (17 French in most patients) to the lower abdominal aorta. To avoid limb ischaemia in smaller patients, two arterial cannulae of 12 French in both femoral arteries were recommended. The system is capable of delivering flow up to 4.0 L/min at 7500 r.p.m.; however, with a bilateral 12 French cannulation the flow is limited to 3.0 L/min. Heparin was administered continuously through the device lubrication system, and the activated clotting time was maintained at 180–200 s.
In patients randomized to standard therapy, an IABP (Datascope Corporation, Fairfield, NJ, USA) was inserted percutaneously according to standard procedures. Heparin was administered intravenous (iv) continuously at the same dose as for the VAD group. All patients were initially on a pumping ratio of 1 : 1 with 100% balloon inflation.
A safety monitoring board (H.T., G.S.) reviewed the ongoing safety data. In case of device related severe complications such as an excess in death and cerebrovascular accidents with neurological dysfunction, the study could be stopped prematurely.
Haemodynamic monitoring
Haemodynamic parameters were acquired in the cath lab before and after device implantation. At the ICU, measurements were obtained every 8 h on subsequent days. The following parameters were measured: cardiac output (mean of three measurements by thermodilution); CI; mean blood pressure (measured by the mean of the arterial line); cardiac power index (CPI); defined as CI×mean arterial pressure×0.0022,9 mean pulmonary artery pressure; PCWP; central venous pressure; and heart rate. In addition, metabolic parameters such as standard base excess, serum lactate, and pH were determined.
Pharmacological treatment
Pharmacological treatment included iv administration of dopamine and dobutamine, if the systemic vascular resistance was high.10 Diuretics and fluids were given on the basis of the estimated optimal filling pressures, according to standard intensive care guidelines.10 In addition, all patients with PCI were started with aspirin 500 mg and clopidogrel 300 mg. This medication was continued for a minimum of 4 weeks with clopidogrel at 75 mg and aspirin indefinitely at 100 mg.
Weaning, device explantation, and 30 day follow-up
Weaning criteria included haemodynamic and clinical evidence that the patient was no longer in CS. Haemodynamic criteria were CI>2.1 L/min/m2 and mean arterial pressure >70 mmHg. Clinical criteria were defined as no inotropic support >12 h and absence of endorgan hypoperfusion. For VAD patients, a stepwise pump output reduction in steps of 500 mL/min every hour was performed according to haemodynamic stability; resulting in a 4–8 h weaning procedure in most patients. Afterwards, the cannulae were removed by surgical approach in the first eight patients. Subsequently, they were removed manually and the puncture site compressed by a compression system (FemoStop® II Plus, RADI Medical Systems, Uppsala, Sweden). For IABP patients, weaning was performed by stepwise reduction of the pumping ratio from 1 : 1 to 1 : 2 and 1 : 3, resulting in a similar weaning time of 4–8 h. The IABP was removed and the puncture site compressed till haemostasis. The 30 day follow-up was performed by outpatient visits or by telephone contact of the patient or the general practitioner. The follow-up was complete.
Endpoints
The pre-specified primary endpoint was defined as haemodynamic improvement, as measured by CPI within 2 h after device implantation. Secondary endpoints were all other haemodynamic and metabolic parameters and the change in haemodynamic and metabolic parameters at longer follow-up. Furthermore, mortality at 30 days and device related complications, such as lower extremity ischaemia requiring surgical or interventional action, cerebrovascular accidents with neurological dysfunction, major bleedings requiring transfusion of blood components, and elevated body temperature >38.5°C, were assessed.
Statistical analysis
The number of patients included was based on the sample size estimation for the primary endpoint. On the basis of the previous studies, we assumed that the absolute change in CPI by VAD would be 1.5±0.5 W/m2 when compared with a change of 0.6±0.2 W/m2 by IABP.4,7 Choosing a power of 90% and a two-sided α-value of 0.05, 20 patients were sufficient in each group.
All analyses were performed according to the intention-to-treat principle. Each categorical variable is expressed as the number and per cent of patients. Continuous parameters were estimated as median and interquartile range (IQR). Differences between treatment groups were assessed by the Fisher's exact or the Chi-square test for categorical variables and by the Student's t-test for continuous data with normal distribution. Otherwise the non-parametric Wilcoxon rank-sum test was used. For mortality the Kaplan–Meier method was used and differences assessed by the log-rank test. A two-tailed P-value <0.05 was considered statistically significant.
Results
Patient characteristics
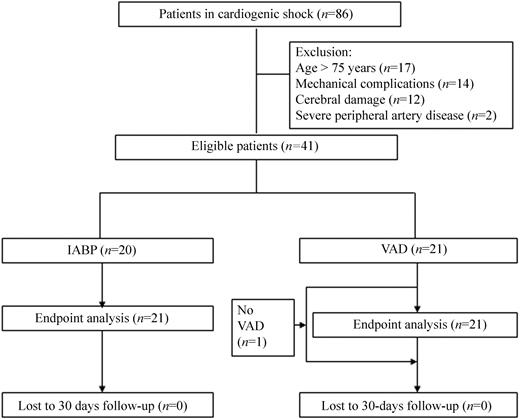
A total of 86 patients were screened for inclusion; 45 patients were excluded (Figure 1). Thus, 20 patients were randomized to IABP and 21 patients to VAD. One patient did not receive the VAD, as the patient showed rapid haemodynamic improvement after PCI (Figure 1). This patient was included in the final analysis, according to the intention-to-treat principle. At the time of enrolment, all patients were in CS according to the defined criteria. The predicted 30 day mortality was 68% in the VAD and 75% in the IABP group. Further patient characteristics are described in Table 1.
Treatment
Treatment was similar with exception of the device employed (Table 2). Only one patient in the VAD group had bilateral arterial cannulation for device implantation. The median times to establish left ventricular assist were longer with 25.0 min (IQR 19.5–27.8) in the VAD in comparison to the IABP group with 11.5 min (IQR 8.5–17.5, P<0.001). The majority of patients (95%) underwent PCI and one patient in each treatment group underwent coronary artery bypass grafting. The implantation procedure for the IABP and VAD was uneventful; in 55% and 57%, revascularization was performed before device implantation.
Haemodynamic characteristics
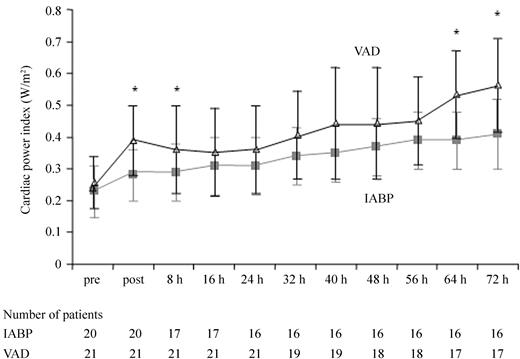
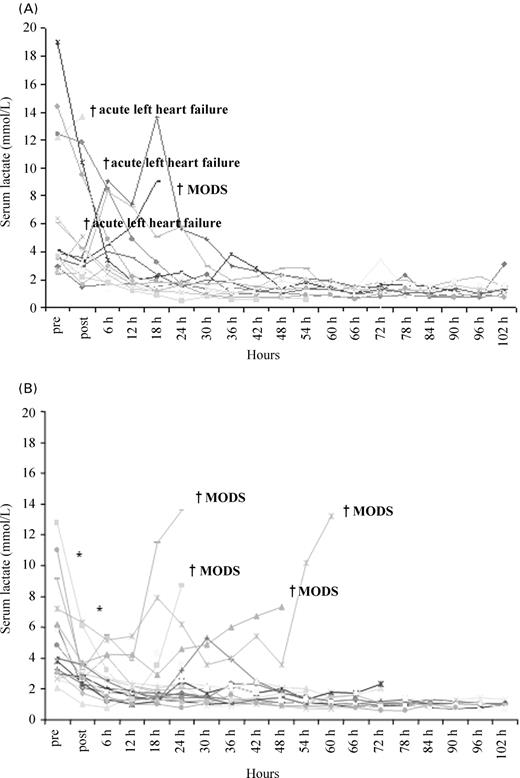
Haemodynamic indices at baseline were similar for both groups, except for a higher PCWP in the IABP group (Table 3). The primary endpoint CPI could be improved more effectively by VAD from 0.22 (IQR 0.19–0.30) to 0.37 W/m2 (IQR 0.30–0.47, P<0.001) when compared with IABP 0.22 (IQR 0.18–0.30) to 0.28 W/m2 (IQR 0.24–0.36, P=0.02; P=0.004 for intergroup comparison). The time of the first CPI measurement after device implantation was similar with 33.5 min (IQR 19.0–50.5) for IABP and 40.0 min (IQR 25.5–53.5, P=0.28) for the VAD. At follow-up (not all patients included in the analysis as a consequence of death), the CPI was higher when compared with IABP at 8, 64, and 72 h (Figure 2). By the VAD, the cardiac output, PCWP, pulmonary artery pressure, and serum lactate could be improved more effectively as by IABP treatment and there was a trend towards improvement for the central venous pressure (Table 3). However, at longer follow-up the time course of lactate was not significantly different between both treatment groups except for the initial and 6 h measurement (Figure 3A and B).
Similar to the haemodynamic improvement, there was an improvement in the renal function measured as urine output per hour from 30 (IQR 20–80) to 80 mL/h (IQR 43–90; P=0.02) for those patients in the VAD group, whereas in the IABP group there was only a slight improvement (pre-IABP 28 mL/h, IQR 0–33; post-IABP 30 mL/h, IQR 25–60, P=0.18; P=0.04 for intergroup comparison).
Clinical course, complications, and outcome
The median VAD flow was 3.4 L/min (IQR 3.0–3.8) with a range of 2.4–3.9 L/min depending on the arterial cannula size and type of cannulation. Median duration of cardiac support was 4.0 days (IQR 3.5–4.0) in the IABP and 3.5 days (IQR 2.0–4.5) in the VAD group (P=0.82). The dose of inotropes, as well as inotropic support time, was similar between both treatment groups (3.0 days, IQR 1.0–3.0 vs. 2.0 days, IQR 1.0–4.0; P=0.52). However, there was a trend towards a longer time on mechanical ventilation for VAD patients (2.5 days, IQR 1.0–5.0 vs. 4.0 days, IQR 2.0–7.0; P=0.07).
In the VAD group, seven patients (IABP n=0; P=0.009) developed limb ischaemia after implantation of a 17 French arterial cannula. Limb ischaemia could be resolved by surgical approach in three patients and by percutaneous approach in four patients. During the follow-up period, 19 patients required transfusions of packed red blood cells in the VAD and eight patients in the IABP group (P=0.002). Patients in the VAD group required 8.0 units (IQR 3.8–16.5) of packed red blood cells vs. 0 units (IQR 0–2.0) (P<0.001) in the IABP group. Fresh frozen plasma and platelets were also required more often in the VAD group (15 vs. 4, P=0.003; and 5 vs. 0, P=0.04). Most of the patients had bleeding at the arterial access site. However, 13 patients in the VAD and 3 patients in the IABP group had signs of disseminated intravascular coagulation (DIC). In the three IABP patients, DIC was rather mild and could be resolved by substitution of antithrombin III. In eight VAD patients, DIC was severe with subsequent haemorrhagic diathesis.
There was a trend towards a higher rate of fever in the VAD group (17 vs. 10, P=0.08). The peak white blood cell count was also higher (19.1, IQR 15.8–24.7 Gpt/L vs. 15.1, IQR 13.8–19.3 Gpt/L, P=0.03) reflecting a higher inflammation rate.
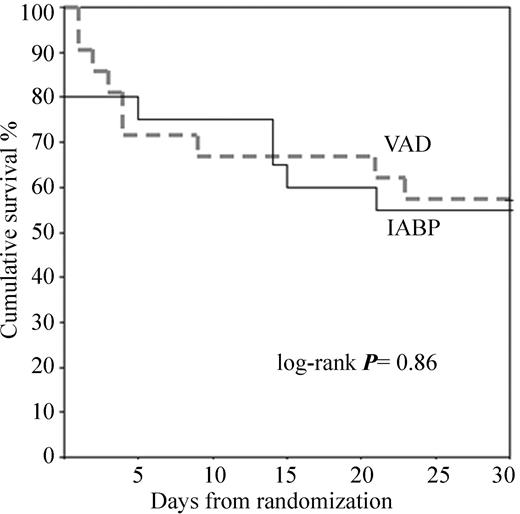
Mortality during support included four patients in each group. In the IABP group, three patients died owing to left heart failure within the first 2 h and one additional patient within 24 h after PCI as a consequence of multiorgan dysfunction syndrome (MODS). In the VAD group, none died within the 24 h. However, four patients died between day 2 and 4 as a cause of MODS despite active circulatory support. Five more patients expired in each group after weaning during 30 day follow-up, resulting in an overall mortality of 45% in the IABP and 43% in the VAD group (log-rank, P=0.86) (Figure 4). In the VAD group, three patients expired after weaning as a cause of recurrent left heart failure, and two as a cause of sepsis or MODS. The cause of death after IABP explantation was MODS in three and acute left heart failure in two patients. There were no 30 day mortality differences for patients with pre-PCI assist support (44% VAD vs. 56% IABP) and for those with post-PCI support (42% VAD vs. 36% IABP).
Discussion
Two important issues emerge from this randomized study in patients with CS complicating AMI: (i) In patients with severe depression of left ventricular performance, circulatory support derived from a VAD is significantly more effective when compared with IABP. (ii) The complications associated with the highly invasive nature of the procedure and the extracorporeal support need to be weighed against the benefits.
Haemodynamic parameters
The best way to characterize any dependant system of pump and pipes is to measure the pumping power of the pump and the flow resistance within the pipes. This is measured by the simplified CPI, which is a comprehensive indicator of cardiac function,11 and a powerful risk stratification tool in acute and chronic heart failure.9,12 Therefore, this parameter has been defined as primary outcome measure. In the current trial, the CPI prior randomization was in the range of those previously reported for patients in severe CS.7,12 The initial effects with an increase of the CPI by >60% by the VAD and an improvement of the other haemodynamic parameters when compared with IABP support were significant. In addition, CS was reversed initially in all patients as monitored by serum lactate. However, partly as a consequence of haemodynamic worsening in some patients in the VAD group and selection owing to death, the haemodynamic effects on CPI were not sustained over time. Furthermore, the CPI rise by a VAD does not necessarily result in improved outcome, as shown by the dissociation of haemodynamic and clinical results in the VAD group. This is mainly reflected by the fact that the CPI rise reflects both the VAD and the heart power.
Clinical course, complications, and outcome
Although this trial did not have the power to detect differences in mortality, there was no trend in mortality benefit for the VAD patients despite the beneficial haemodynamic effects. One factor might be the development of a systemic inflammatory response syndrome (SIRS), which occurs in non-infectious diseases such as large AMI.13 SIRS may be initiated by a number of processes in CS, including blood contact with foreign surfaces, ischaemia and the reperfusion injury by PCI, and the presence of endotoxemia.14,15 Extracorporeal circulation might be deleterious by further activation of inflammatory cytokines, which act as a potent stimulus for toxic levels of NO and peroxynitrite production with subsequent impaired myocardial contractility, pathological peripheral vasodilatation, proinflammatory effects, and reduced catecholamine response.16 Pharmacological blocking of NO production has shown beneficial effects in small trials.17 As a consequence, the extracorporeal VAD might have initial beneficial effects on haemodynamic parameters, but might further promote SIRS with subsequent deterioration to MODS. The higher rate of fever and white blood cell counts in VAD patients support this hypothesis.
A second deleterious effect of extracorporeal circulation besides the propagation of SIRS is the activation of complement and the development of coagulation with subsequent fibrinolysis, which may progress to DIC leading to severe bleeding complications.15,18 Nearly, all patients treated >2 days by VAD support required blood transfusion as a consequence of DIC, supporting this pathophysiological interaction.19
A significant number of patients developed distal limb ischaemia due to the large arterial cannula, which is imperative for VAD effectiveness. However, this problem could be dealt in most of the patients by percutaneous insertion of an additional downstream perfusion cannula.
In contrast to the initial feasibility study, embolic events did not occur in patients treated either by VAD or by IABP, which may be accounted to the careful monitored anticoagulation regimen.
Another important issue is the VAD effect on coronary flow. Depending on left ventricular unloading, the coronary flow might be totally laminar in patients supported by a left atrial-to-femoral arterial VAD in contrast to IABP support. This is unphysiological and might be deleterious. However, currently little is known and the effects of different flow patterns are under intensive debate.20–22
Effects of left ventricular unloading
Besides the haemodynamic effects of an active VAD in comparison to IABP, the recovery of myocardium after revascularization occurs by a reduction of the filling pressure in the left ventricle, cardiac workload, and oxygen demand. Previous animal studies have demonstrated a decrease in infarct size by the use of VADs with or without revascularization.23–25 Furthermore, timing of unloading prior to revascularization might have beneficial effects with an additional reduction of infarct size.26 However, a clinical trial supporting this hypothesis is pending. Therefore, in the current trial, timing of left ventricular unloading and revascularization of the infarcted vessel was left to the discretion of the operator. A sub-analysis of those patients unloaded prior to revascularization showed no differences in outcome to those unloaded after revascularization.
Mortality probability
Risk stratification in CS patients is an important tool for a carefully tailored treatment strategy with a more aggressive approach, including PCI and VAD support, in very high risk patients and a moderate aggressive approach, such as PCI plus IABP, in low or intermediate risk patients. Therefore, a reliable prognostic score is of paramount importance. The mortality probability score used in this trial for the assessment of 30 day mortality has been evaluated and confirmed in several thousand patients in the thrombolytic-era.8 Since the introduction of PCI as standard care in CS patients,27 a prognostic score incorporating demographic, clinical, haemodynamic, and angiographic data has not been prospectively evaluated. Therefore, a new score warrants establishment in the PCI-era. The clinical variables in the current trial were all indicators for high mortality with a high proportion of patients with acute renal failure. Thus, the predicted mortality was high. However, final mortality in both groups was much lower and comparable to the early PCI group in the SHOCK trial.27 On the basis of the results of the present trial, a stepwise strategy could be formulated with respect to IABP or VAD utilization. All patients with CS should initially be treated by IABP. In those not responding sufficiently, the insertion of a VAD in addition to IABP might be considered. This would prevent patients responding favourably to IABP alone to be exposed to a more invasive procedure.
Newer devices for short-term support with less invasive implantation procedure, smaller cannula, and without extracorporeal support might be the solution for the high VAD associated morbidity. However, so far these have to be developed or their haemodynamic effectiveness and durability remain unproved.23
Study limitations
As the study was conducted in a tertiary care centre, this might preclude generalization of the results to standard care of CS patients. The small number of patients studied precludes from a multivariate analysis to draw conclusions, which patients can be stabilized by either IABP or VAD. Furthermore, the exclusion criteria eliminated >50% of the patients in CS. Thus, the results of the current trial cannot be generalized to the entire CS population. These exclusion criteria have been determined, as the benefit of an early PCI approach in older patients is questionable6,27 and as patients with mechanical complications following an AMI have a different prognosis.28 Finally, a longer follow-up will be needed to address long-term efficacy.
Conclusions
The haemodynamic and metabolic parameters in CS can be reversed more effectively by a VAD with active circulatory support as by standard IABP treatment. However, there were more complications encountered by the highly invasive procedure and the extracorporeal support. The decision-making process on how to treat CS in addition to PCI and inotropes requires an integrated stepwise approach based on the individual risk, success rates, and periprocedural event rates of the support device.
Acknowledgements
The study was supported in part by a research grant from Cardiac Assist, Pittsburgh, PA, USA. We thank all physicians and nurses at our ICU. Furthermore, we are indebted to all perfusionists for technical assistance during VAD implantation.
Figure 1 Trial profile.
Figure 2 Time course of CPI at follow-up for IABP and VAD. Asterisk indicates P<0.05 for the intergroup comparison of the CPI measured in the IABP vs. VAD group at different time points.
Figure 3 (A) Time course of serum lactate for individual patients treated by IABP. (B) Time course of serum lactate for individual patients treated by VAD. Asterisk indicates P<0.05 for the intergroup comparison of serum lactate for the VAD vs. IABP group measured directly after and 6 h after implantation.
Figure 4 Kaplan–Meier survival estimates for 30 day survival for IABP and VAD.
Patient characteristics
| . | IABP (n=20) . | VAD (n=21) . | P-value . |
|---|---|---|---|
| Age (years) | 65 (59–73) | 63 (57–70) | 0.21 |
| Male, n (%) | 15 (75) | 16 (76) | 1.0 |
| Height (cm) | 173 (168–179) | 173 (169–177) | 1.0 |
| Hypertension, n (%) | 15 (75) | 19 (90) | 0.24 |
| Diabetes mellitus, n (%) | 11 (55) | 11 (52) | 0.89 |
| Smoking, n (%) | 6 (30) | 9 (43) | 0.60 |
| Hypercholesterolemia, n (%) | 9 (45) | 11 (52) | 0.87 |
| Prior MI, n (%) | 9 (45) | 13 (62) | 0.44 |
| Anterior index MI, n (%) | 13 (65) | 18 (86) | 0.16 |
| Peak creatine kinase (U/L) | 4186 (1913–7296) | 5442 (2272–8073) | 0.96 |
| Prior thrombolysis, n (%) | 6 (30) | 10 (48) | 0.40 |
| Time from AMI to randomization (h) | 10.0 (5.3–25.5) | 11.0 (6.8–18.8) | 0.92 |
| Time from AMI to shock (h) | 4.5 (2.5–17.5) | 5.0 (2.8–11.3) | 0.94 |
| Left ventricular ejection fraction (%) | 28.5 (20.5–30.5) | 25.0 (20.0–32.8) | 0.83 |
| Number of diseased vessels (Per cent stenosis >50%), n (%) | 0.39 | ||
| 1 | 6 (30) | 8 (38) | |
| 2 | 4 (20) | 4 (19) | |
| 3 | 10 (50) | 9 (43) | |
| Left main | 1 (5) | 2 (10) | |
| Urine output <30 mL/h, n (%) | 15 (75) | 10 (48) | 0.14 |
| Baseline renal function (mL/h) | 27.5 (0–32.5) | 30.0 (20.0–80.0) | 0.29 |
| . | IABP (n=20) . | VAD (n=21) . | P-value . |
|---|---|---|---|
| Age (years) | 65 (59–73) | 63 (57–70) | 0.21 |
| Male, n (%) | 15 (75) | 16 (76) | 1.0 |
| Height (cm) | 173 (168–179) | 173 (169–177) | 1.0 |
| Hypertension, n (%) | 15 (75) | 19 (90) | 0.24 |
| Diabetes mellitus, n (%) | 11 (55) | 11 (52) | 0.89 |
| Smoking, n (%) | 6 (30) | 9 (43) | 0.60 |
| Hypercholesterolemia, n (%) | 9 (45) | 11 (52) | 0.87 |
| Prior MI, n (%) | 9 (45) | 13 (62) | 0.44 |
| Anterior index MI, n (%) | 13 (65) | 18 (86) | 0.16 |
| Peak creatine kinase (U/L) | 4186 (1913–7296) | 5442 (2272–8073) | 0.96 |
| Prior thrombolysis, n (%) | 6 (30) | 10 (48) | 0.40 |
| Time from AMI to randomization (h) | 10.0 (5.3–25.5) | 11.0 (6.8–18.8) | 0.92 |
| Time from AMI to shock (h) | 4.5 (2.5–17.5) | 5.0 (2.8–11.3) | 0.94 |
| Left ventricular ejection fraction (%) | 28.5 (20.5–30.5) | 25.0 (20.0–32.8) | 0.83 |
| Number of diseased vessels (Per cent stenosis >50%), n (%) | 0.39 | ||
| 1 | 6 (30) | 8 (38) | |
| 2 | 4 (20) | 4 (19) | |
| 3 | 10 (50) | 9 (43) | |
| Left main | 1 (5) | 2 (10) | |
| Urine output <30 mL/h, n (%) | 15 (75) | 10 (48) | 0.14 |
| Baseline renal function (mL/h) | 27.5 (0–32.5) | 30.0 (20.0–80.0) | 0.29 |
Values of continuous variables are given as median (IQR).
Patient characteristics
| . | IABP (n=20) . | VAD (n=21) . | P-value . |
|---|---|---|---|
| Age (years) | 65 (59–73) | 63 (57–70) | 0.21 |
| Male, n (%) | 15 (75) | 16 (76) | 1.0 |
| Height (cm) | 173 (168–179) | 173 (169–177) | 1.0 |
| Hypertension, n (%) | 15 (75) | 19 (90) | 0.24 |
| Diabetes mellitus, n (%) | 11 (55) | 11 (52) | 0.89 |
| Smoking, n (%) | 6 (30) | 9 (43) | 0.60 |
| Hypercholesterolemia, n (%) | 9 (45) | 11 (52) | 0.87 |
| Prior MI, n (%) | 9 (45) | 13 (62) | 0.44 |
| Anterior index MI, n (%) | 13 (65) | 18 (86) | 0.16 |
| Peak creatine kinase (U/L) | 4186 (1913–7296) | 5442 (2272–8073) | 0.96 |
| Prior thrombolysis, n (%) | 6 (30) | 10 (48) | 0.40 |
| Time from AMI to randomization (h) | 10.0 (5.3–25.5) | 11.0 (6.8–18.8) | 0.92 |
| Time from AMI to shock (h) | 4.5 (2.5–17.5) | 5.0 (2.8–11.3) | 0.94 |
| Left ventricular ejection fraction (%) | 28.5 (20.5–30.5) | 25.0 (20.0–32.8) | 0.83 |
| Number of diseased vessels (Per cent stenosis >50%), n (%) | 0.39 | ||
| 1 | 6 (30) | 8 (38) | |
| 2 | 4 (20) | 4 (19) | |
| 3 | 10 (50) | 9 (43) | |
| Left main | 1 (5) | 2 (10) | |
| Urine output <30 mL/h, n (%) | 15 (75) | 10 (48) | 0.14 |
| Baseline renal function (mL/h) | 27.5 (0–32.5) | 30.0 (20.0–80.0) | 0.29 |
| . | IABP (n=20) . | VAD (n=21) . | P-value . |
|---|---|---|---|
| Age (years) | 65 (59–73) | 63 (57–70) | 0.21 |
| Male, n (%) | 15 (75) | 16 (76) | 1.0 |
| Height (cm) | 173 (168–179) | 173 (169–177) | 1.0 |
| Hypertension, n (%) | 15 (75) | 19 (90) | 0.24 |
| Diabetes mellitus, n (%) | 11 (55) | 11 (52) | 0.89 |
| Smoking, n (%) | 6 (30) | 9 (43) | 0.60 |
| Hypercholesterolemia, n (%) | 9 (45) | 11 (52) | 0.87 |
| Prior MI, n (%) | 9 (45) | 13 (62) | 0.44 |
| Anterior index MI, n (%) | 13 (65) | 18 (86) | 0.16 |
| Peak creatine kinase (U/L) | 4186 (1913–7296) | 5442 (2272–8073) | 0.96 |
| Prior thrombolysis, n (%) | 6 (30) | 10 (48) | 0.40 |
| Time from AMI to randomization (h) | 10.0 (5.3–25.5) | 11.0 (6.8–18.8) | 0.92 |
| Time from AMI to shock (h) | 4.5 (2.5–17.5) | 5.0 (2.8–11.3) | 0.94 |
| Left ventricular ejection fraction (%) | 28.5 (20.5–30.5) | 25.0 (20.0–32.8) | 0.83 |
| Number of diseased vessels (Per cent stenosis >50%), n (%) | 0.39 | ||
| 1 | 6 (30) | 8 (38) | |
| 2 | 4 (20) | 4 (19) | |
| 3 | 10 (50) | 9 (43) | |
| Left main | 1 (5) | 2 (10) | |
| Urine output <30 mL/h, n (%) | 15 (75) | 10 (48) | 0.14 |
| Baseline renal function (mL/h) | 27.5 (0–32.5) | 30.0 (20.0–80.0) | 0.29 |
Values of continuous variables are given as median (IQR).
Treatment
| . | IABP (n=20) . | VAD (n=21) . | P-value . |
|---|---|---|---|
| CPR, VT, or VF before randomization | 11 (55) | 11 (52) | 0.89 |
| Inotropes or vasopressors | 20 (100) | 21 (100) | 1.00 |
| Mechanical ventilation | 20 (100) | 20 (95) | 1.00 |
| PCI (plus stenting) | 19 (95) | 20 (95) | 1.00 |
| TIMI flow pre-percutaneous PCI | 0.41 | ||
| 0 | 10 (53) | 12 (60) | |
| 1 | 1 (5) | 2 (10) | |
| 2 | 1 (5) | 2 (10) | |
| 3 | 7 (37) | 4 (20) | |
| TIMI flow post-PCI | 0.39 | ||
| 0 | 1 (5) | 0 | |
| 1 | 1 (5) | 1 (5) | |
| 2 | 3 (16) | 3 (15) | |
| 3 | 14 (74) | 16 (80) | |
| Platelet glycoprotein IIb/IIIa antagonist | 7 (35) | 7 (33) | 0.83 |
| PCI before assisted circulation | 11 (55) | 12 (57) | 0.86 |
| CABG | 1 (5) | 1 (5) | 1.00 |
| . | IABP (n=20) . | VAD (n=21) . | P-value . |
|---|---|---|---|
| CPR, VT, or VF before randomization | 11 (55) | 11 (52) | 0.89 |
| Inotropes or vasopressors | 20 (100) | 21 (100) | 1.00 |
| Mechanical ventilation | 20 (100) | 20 (95) | 1.00 |
| PCI (plus stenting) | 19 (95) | 20 (95) | 1.00 |
| TIMI flow pre-percutaneous PCI | 0.41 | ||
| 0 | 10 (53) | 12 (60) | |
| 1 | 1 (5) | 2 (10) | |
| 2 | 1 (5) | 2 (10) | |
| 3 | 7 (37) | 4 (20) | |
| TIMI flow post-PCI | 0.39 | ||
| 0 | 1 (5) | 0 | |
| 1 | 1 (5) | 1 (5) | |
| 2 | 3 (16) | 3 (15) | |
| 3 | 14 (74) | 16 (80) | |
| Platelet glycoprotein IIb/IIIa antagonist | 7 (35) | 7 (33) | 0.83 |
| PCI before assisted circulation | 11 (55) | 12 (57) | 0.86 |
| CABG | 1 (5) | 1 (5) | 1.00 |
CPR, cardiopulmonary resuscitation; VT, sustained ventricular tachycardia; VF, ventricular fibrillation; CABG, coronary artery bypass grafting. Values presented as n (%).
Treatment
| . | IABP (n=20) . | VAD (n=21) . | P-value . |
|---|---|---|---|
| CPR, VT, or VF before randomization | 11 (55) | 11 (52) | 0.89 |
| Inotropes or vasopressors | 20 (100) | 21 (100) | 1.00 |
| Mechanical ventilation | 20 (100) | 20 (95) | 1.00 |
| PCI (plus stenting) | 19 (95) | 20 (95) | 1.00 |
| TIMI flow pre-percutaneous PCI | 0.41 | ||
| 0 | 10 (53) | 12 (60) | |
| 1 | 1 (5) | 2 (10) | |
| 2 | 1 (5) | 2 (10) | |
| 3 | 7 (37) | 4 (20) | |
| TIMI flow post-PCI | 0.39 | ||
| 0 | 1 (5) | 0 | |
| 1 | 1 (5) | 1 (5) | |
| 2 | 3 (16) | 3 (15) | |
| 3 | 14 (74) | 16 (80) | |
| Platelet glycoprotein IIb/IIIa antagonist | 7 (35) | 7 (33) | 0.83 |
| PCI before assisted circulation | 11 (55) | 12 (57) | 0.86 |
| CABG | 1 (5) | 1 (5) | 1.00 |
| . | IABP (n=20) . | VAD (n=21) . | P-value . |
|---|---|---|---|
| CPR, VT, or VF before randomization | 11 (55) | 11 (52) | 0.89 |
| Inotropes or vasopressors | 20 (100) | 21 (100) | 1.00 |
| Mechanical ventilation | 20 (100) | 20 (95) | 1.00 |
| PCI (plus stenting) | 19 (95) | 20 (95) | 1.00 |
| TIMI flow pre-percutaneous PCI | 0.41 | ||
| 0 | 10 (53) | 12 (60) | |
| 1 | 1 (5) | 2 (10) | |
| 2 | 1 (5) | 2 (10) | |
| 3 | 7 (37) | 4 (20) | |
| TIMI flow post-PCI | 0.39 | ||
| 0 | 1 (5) | 0 | |
| 1 | 1 (5) | 1 (5) | |
| 2 | 3 (16) | 3 (15) | |
| 3 | 14 (74) | 16 (80) | |
| Platelet glycoprotein IIb/IIIa antagonist | 7 (35) | 7 (33) | 0.83 |
| PCI before assisted circulation | 11 (55) | 12 (57) | 0.86 |
| CABG | 1 (5) | 1 (5) | 1.00 |
CPR, cardiopulmonary resuscitation; VT, sustained ventricular tachycardia; VF, ventricular fibrillation; CABG, coronary artery bypass grafting. Values presented as n (%).
Haemodynamic parameters pre- and post-IABP and VAD implantation
| . | Pre-implantation IABP . | Pre-implantation VAD . | P-value . | Post-implantation IABP . | Post-implantation VAD . | P-value . |
|---|---|---|---|---|---|---|
| Cardiac output (L/min) | 3.0 (2.5–4.0) | 3.5 (3.3–4.2) | 0.29 | 3.3 (2.9–4.3) | 4.5 (4.0–5.4) | 0.007 |
| CI (L/min/m2) | 1.5 (1.3–2.0) | 1.7 (1.5–2.1) | 0.35 | 1.7 (1.5–2.1) | 2.3 (1.9–2.7) | 0.005 |
| Blood pressure mean (mmHg) | 64 (57–74) | 63 (51–70) | 0.50 | 67 (62–84) | 74 (70–84) | 0.38 |
| CPI (W/m2) | 0.22 (0.18–0.30) | 0.22 (0.19–0.30) | 0.72 | 0.28 (0.24–0.36) | 0.37 (0.30–0.47) | 0.004 |
| SVR (dyn×s×cm−5) | 1440 (1034–1758) | 1049 (852–1284) | 0.16 | 1388 (998–1809) | 1153 (844–1425) | 0.08 |
| Heart rate (beats/min) | 122 (92–130) | 113 (107–121) | 0.57 | 115 (90–125) | 105 (100–116) | 0.94 |
| PCWP (mmHg) | 27.0 (20.0–30.0) | 20.0 (18.0–23.0) | 0.02 | 21.5 (17.0–26.0) | 16.0 (12.5–19.0) | 0.003 |
| Central venous pressure (mmHg) | 13.0 (11.0–16.5) | 11.0 (9.0–15.3) | 0.29 | 12.0 (10.0–17.5) | 10.0 (8.0–12.0) | 0.06 |
| PAP mean (mmHg) | 32.5 (27.5–38.0) | 28.0 (24.5–34.8) | 0.45 | 28.5 (25.5–33.5) | 24.5 (20.0–26.0) | 0.007 |
| Serum lactate (mmol/L) | 3.8 (3.5–6.7) | 4.5 (3.1–6.5) | 0.53 | 3.25 (2.7–7.0) | 2.8 (2.3–3.5) | 0.03 |
| Standard base excess (mmol/L) | −6.8 [−8.3–(−3.9)] | −5.1 [−7.5–(−4.4)] | 0.74 | −4.3 [−8.8–(−2.3)] | −4.3 [−6.1–(−3.3)] | 0.28 |
| pH | 7.34 (7.28–7.38) | 7.28 (7.24–7.36) | 0.50 | 7.36 (7.28–7.41) | 7.33 (7.31–7.40) | 0.49 |
| . | Pre-implantation IABP . | Pre-implantation VAD . | P-value . | Post-implantation IABP . | Post-implantation VAD . | P-value . |
|---|---|---|---|---|---|---|
| Cardiac output (L/min) | 3.0 (2.5–4.0) | 3.5 (3.3–4.2) | 0.29 | 3.3 (2.9–4.3) | 4.5 (4.0–5.4) | 0.007 |
| CI (L/min/m2) | 1.5 (1.3–2.0) | 1.7 (1.5–2.1) | 0.35 | 1.7 (1.5–2.1) | 2.3 (1.9–2.7) | 0.005 |
| Blood pressure mean (mmHg) | 64 (57–74) | 63 (51–70) | 0.50 | 67 (62–84) | 74 (70–84) | 0.38 |
| CPI (W/m2) | 0.22 (0.18–0.30) | 0.22 (0.19–0.30) | 0.72 | 0.28 (0.24–0.36) | 0.37 (0.30–0.47) | 0.004 |
| SVR (dyn×s×cm−5) | 1440 (1034–1758) | 1049 (852–1284) | 0.16 | 1388 (998–1809) | 1153 (844–1425) | 0.08 |
| Heart rate (beats/min) | 122 (92–130) | 113 (107–121) | 0.57 | 115 (90–125) | 105 (100–116) | 0.94 |
| PCWP (mmHg) | 27.0 (20.0–30.0) | 20.0 (18.0–23.0) | 0.02 | 21.5 (17.0–26.0) | 16.0 (12.5–19.0) | 0.003 |
| Central venous pressure (mmHg) | 13.0 (11.0–16.5) | 11.0 (9.0–15.3) | 0.29 | 12.0 (10.0–17.5) | 10.0 (8.0–12.0) | 0.06 |
| PAP mean (mmHg) | 32.5 (27.5–38.0) | 28.0 (24.5–34.8) | 0.45 | 28.5 (25.5–33.5) | 24.5 (20.0–26.0) | 0.007 |
| Serum lactate (mmol/L) | 3.8 (3.5–6.7) | 4.5 (3.1–6.5) | 0.53 | 3.25 (2.7–7.0) | 2.8 (2.3–3.5) | 0.03 |
| Standard base excess (mmol/L) | −6.8 [−8.3–(−3.9)] | −5.1 [−7.5–(−4.4)] | 0.74 | −4.3 [−8.8–(−2.3)] | −4.3 [−6.1–(−3.3)] | 0.28 |
| pH | 7.34 (7.28–7.38) | 7.28 (7.24–7.36) | 0.50 | 7.36 (7.28–7.41) | 7.33 (7.31–7.40) | 0.49 |
SVR, systemic vascular resistance; PAP, pulmonary artery pressure; values are given as median (IQR).
Haemodynamic parameters pre- and post-IABP and VAD implantation
| . | Pre-implantation IABP . | Pre-implantation VAD . | P-value . | Post-implantation IABP . | Post-implantation VAD . | P-value . |
|---|---|---|---|---|---|---|
| Cardiac output (L/min) | 3.0 (2.5–4.0) | 3.5 (3.3–4.2) | 0.29 | 3.3 (2.9–4.3) | 4.5 (4.0–5.4) | 0.007 |
| CI (L/min/m2) | 1.5 (1.3–2.0) | 1.7 (1.5–2.1) | 0.35 | 1.7 (1.5–2.1) | 2.3 (1.9–2.7) | 0.005 |
| Blood pressure mean (mmHg) | 64 (57–74) | 63 (51–70) | 0.50 | 67 (62–84) | 74 (70–84) | 0.38 |
| CPI (W/m2) | 0.22 (0.18–0.30) | 0.22 (0.19–0.30) | 0.72 | 0.28 (0.24–0.36) | 0.37 (0.30–0.47) | 0.004 |
| SVR (dyn×s×cm−5) | 1440 (1034–1758) | 1049 (852–1284) | 0.16 | 1388 (998–1809) | 1153 (844–1425) | 0.08 |
| Heart rate (beats/min) | 122 (92–130) | 113 (107–121) | 0.57 | 115 (90–125) | 105 (100–116) | 0.94 |
| PCWP (mmHg) | 27.0 (20.0–30.0) | 20.0 (18.0–23.0) | 0.02 | 21.5 (17.0–26.0) | 16.0 (12.5–19.0) | 0.003 |
| Central venous pressure (mmHg) | 13.0 (11.0–16.5) | 11.0 (9.0–15.3) | 0.29 | 12.0 (10.0–17.5) | 10.0 (8.0–12.0) | 0.06 |
| PAP mean (mmHg) | 32.5 (27.5–38.0) | 28.0 (24.5–34.8) | 0.45 | 28.5 (25.5–33.5) | 24.5 (20.0–26.0) | 0.007 |
| Serum lactate (mmol/L) | 3.8 (3.5–6.7) | 4.5 (3.1–6.5) | 0.53 | 3.25 (2.7–7.0) | 2.8 (2.3–3.5) | 0.03 |
| Standard base excess (mmol/L) | −6.8 [−8.3–(−3.9)] | −5.1 [−7.5–(−4.4)] | 0.74 | −4.3 [−8.8–(−2.3)] | −4.3 [−6.1–(−3.3)] | 0.28 |
| pH | 7.34 (7.28–7.38) | 7.28 (7.24–7.36) | 0.50 | 7.36 (7.28–7.41) | 7.33 (7.31–7.40) | 0.49 |
| . | Pre-implantation IABP . | Pre-implantation VAD . | P-value . | Post-implantation IABP . | Post-implantation VAD . | P-value . |
|---|---|---|---|---|---|---|
| Cardiac output (L/min) | 3.0 (2.5–4.0) | 3.5 (3.3–4.2) | 0.29 | 3.3 (2.9–4.3) | 4.5 (4.0–5.4) | 0.007 |
| CI (L/min/m2) | 1.5 (1.3–2.0) | 1.7 (1.5–2.1) | 0.35 | 1.7 (1.5–2.1) | 2.3 (1.9–2.7) | 0.005 |
| Blood pressure mean (mmHg) | 64 (57–74) | 63 (51–70) | 0.50 | 67 (62–84) | 74 (70–84) | 0.38 |
| CPI (W/m2) | 0.22 (0.18–0.30) | 0.22 (0.19–0.30) | 0.72 | 0.28 (0.24–0.36) | 0.37 (0.30–0.47) | 0.004 |
| SVR (dyn×s×cm−5) | 1440 (1034–1758) | 1049 (852–1284) | 0.16 | 1388 (998–1809) | 1153 (844–1425) | 0.08 |
| Heart rate (beats/min) | 122 (92–130) | 113 (107–121) | 0.57 | 115 (90–125) | 105 (100–116) | 0.94 |
| PCWP (mmHg) | 27.0 (20.0–30.0) | 20.0 (18.0–23.0) | 0.02 | 21.5 (17.0–26.0) | 16.0 (12.5–19.0) | 0.003 |
| Central venous pressure (mmHg) | 13.0 (11.0–16.5) | 11.0 (9.0–15.3) | 0.29 | 12.0 (10.0–17.5) | 10.0 (8.0–12.0) | 0.06 |
| PAP mean (mmHg) | 32.5 (27.5–38.0) | 28.0 (24.5–34.8) | 0.45 | 28.5 (25.5–33.5) | 24.5 (20.0–26.0) | 0.007 |
| Serum lactate (mmol/L) | 3.8 (3.5–6.7) | 4.5 (3.1–6.5) | 0.53 | 3.25 (2.7–7.0) | 2.8 (2.3–3.5) | 0.03 |
| Standard base excess (mmol/L) | −6.8 [−8.3–(−3.9)] | −5.1 [−7.5–(−4.4)] | 0.74 | −4.3 [−8.8–(−2.3)] | −4.3 [−6.1–(−3.3)] | 0.28 |
| pH | 7.34 (7.28–7.38) | 7.28 (7.24–7.36) | 0.50 | 7.36 (7.28–7.41) | 7.33 (7.31–7.40) | 0.49 |
SVR, systemic vascular resistance; PAP, pulmonary artery pressure; values are given as median (IQR).
References
Barron HV, Every NR, Parsons LS et al. The use of intra-aortic balloon counterpulsation in patients with cardiogenic shock complicating acute myocardial infarction: data from the National Registry of Myocardial Infarction 2.
Hochman JS, Buller CE, Sleeper LA et al. Cardiogenic shock complicating acute myocardial infarction—etiologies, management and outcome: a report from the SHOCK trial registry.
Goldberg RJ, Samad NA, Yarzebski J et al. Temporal trends in cardiogenic shock complicating acute myocardial infarction.
Scheidt S, Wilner G, Mueller H et al. Intra-aortic balloon counterpulsation in cardiogenic shock. Report of a co-operative clinical trial.
DeWood MA, Notske RN, Hensley GR et al. Intraaortic balloon counterpulsation with and without reperfusion for myocardial infarction shock.
Antman EM, Anbe DT, Armstrong PW et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary.
Thiele H, Lauer B, Hambrecht R et al. Reversal of cardiogenic shock by percutaneous left-atrial-to-femoral arterial bypass assistance.
Hasdai D, Holmes DR Jr, Califf RM et al. Cardiogenic shock complicating acute myocardial infarction: predictors of death.
Williams SG, Cooke GA, Wright DJ et al. Peak exercise cardiac power output: a direct indicator of cardiac function strongly predictive of prognosis in chronic heart failure.
Kellum JA, Pinsky MR. Use of vasopressore agents in critically ill patients.
Tan LB. Clinical and research implications of new concepts in the assessment of cardiac pumping performance in heart failure.
Cotter G, Moshkovitz Y, Kaluski E et al. The role of cardiac power and systemic vascular resistance in the pathophysiology and diagnosis of patients with acute congestive heart failure.
Neumann FJ, Ott I, Gawaz M et al. Cardiac release of cytokines and inflammatory responses in acute myocardial infarction.
Hall RI, Smith MS, Rocker G. The systemic inflammatory response to cardiopulmonary bypass: pathophysiological, therapeutic, and pharmacological considerations.
Hochman JS. Cardiogenic shock complicating acute myocardial infarction. Expanding the paradigm.
Cotter G, Kaluski E, Milo O et al. LINCS: L-NAME (a NO synthase inhibitor) in the treatment of refractory cardiogenic shock.
Kirklin JK, Westaby S, Blackstone EH et al. Complement and the damaging effects of cardiopulmonary bypass.
Gando S, Kameue T, Nanzaki S et al. Disseminated intravascular coagulation is a frequent complication of systemic inflammatory response syndrome.
Finlayson D. Con: nonpulsatile flow is preferable to pulsatile flow during cardiopulmonary bypass.
Shevde K, DeBois W. Pro: nonpulsatile flow is preferable to pulsatile flow during cardiopulmonary bypass.
Potapov EV, Loebe M, Nasseri BA et al. Pulsatile flow in patients with a novel nonpulsatile implantable ventricular assist device.
Meyns B, Stolinski J, Leunens V et al. Left ventricular support by catheter-mounted axial flow pump reduces infarct size.
Laschinger J, Grossi E, Cunningham J et al. Adjunctive left ventricular unloading during myocardial reperfusion plays a major role in minimizing myocardial infarct size.
Smalling RW, Cassidy DB, Barrett R et al. Improved regional myocardial blood flow, left ventricular unloading, and infarct salvage using an axial-flow, transvalvular left ventricular assist device. A comparison with intra-aortic balloon counterpulsation and reperfusion alone in a canine infarction model.
Allen BS, Okamoto F, Buckberg GD et al. Reperfusion conditions: critical importance of total ventricular decompression during regional reperfusion.
Hochman JS, Sleeper LA, Webb JG et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock.