-
PDF
- Split View
-
Views
-
Cite
Cite
Vojtech Melenovsky, Ilan Hay, Barry J. Fetics, Barry A. Borlaug, Andrew Kramer, Joseph M. Pastore, Ronald Berger, David A. Kass, Functional impact of rate irregularity in patients with heart failure and atrial fibrillation receiving cardiac resynchronization therapy, European Heart Journal, Volume 26, Issue 7, April 2005, Pages 705–711, https://doi.org/10.1093/eurheartj/ehi066
Close - Share Icon Share
Abstract
Aims Atrial fibrillation (AFib) with a rapid ventricular response may adversely impact cardiac performance, especially in patients with heart failure. However, it remains uncertain whether rhythm irregularity per se has unfavourable effects apart from tachycardia, and whether rate regularization alone can improve heart function.
Methods and results Nine subjects with chronic AFib, atrioventricular nodal block, and symptomatic heart failure (ejection fraction 14–30%) were studied using a pressure–volume catheter. Ventricles were biventricularly paced (RV-apex, LV-lateral wall) at 80 or 120 min−1 mean rate, using regular or irregular, Poisson-distributed stimulation. At 80 min−1, ventricular function was similar between the two pacing modes. However, at 120 min−1, irregular pacing impaired systolic (dP/dtmax: −8.2%, P<0.001) and diastolic function (dP/dtmin: +21%, P<0.001, LV end-diastolic pressure: +26%, P=0.007) compared with regular rate pacing. Contractile function during irregular pacing varied with the ratio of preceding/pre-preceding intercycle (RR) interval (dP/dtmax: 80 b.p.m.: r=0.69; 120 b.p.m.: r=0.74), whereas pre-load had little effect on instantaneous contractility.
Conclusion In heart failure subjects with AFib, RR-interval irregularity worsens cardiac function at elevated but not at normal range heart rate. Overall rate control is most important in these patients while rate regularization of rapid AFib may impart additional benefits.
See page 637 for the editorial comment on this article (doi:10.1093/eurheartj/ehi234)
Introduction
Atrial fibrillation (AFib) adversely affects cardiac haemodynamics by several mechanisms. Loss of atrial contraction can limit net filling and delay mitral valve closure augmenting pre-systolic regurgitation.1 Diastolic filling is further impaired by short cycle lengths with rapid AFib, while persistent rate elevation can trigger systolic depression. Studies further suggest that rate irregularity itself may negatively impact cardiac performance.1–3 Rate regularization can be achieved with overdrive right ventricular (RV)-pacing; however, any benefits are countered by the adverse effects of pacing-induced dys-synchrony.4,5 Biventricular or left ventricular (LV) free-wall pacing6 can diminish the latter, but such therapies are complex, making it important to determine whether there is indeed a net benefit from rate regularization and/or under what conditions it might occur.
Changes in ventricular performance with cycle length are due in part to varying diastolic filling time and to the force–frequency relation (FFR) that couples stimulation rate to contractile function.7,8 Beat-to-beat variability in cycle length (as with AFib) further affects both filling and contractility as a given beat can be premature relative to the prior cycle length (lower contractility and pre-load)or potentiated (opposite).9–11 Both the FFR and rate dependence of chamber filling are altered by dilated heart failure;7 the former from abnormalities in calciumcycling12 and the latter due to depressed function and chamber remodelling. Whether this influences the impact of rate irregularity on net cardiac function remains unknown.
Accordingly, the present study tested whether rate irregularity itself is detrimental to LV function in patients with chronic AFib and heart failure, and directly examined the haemodynamic impact of rate regularization through biventricular pacing. We further tested whether the impact of rate regularization depends on mean heart rate and identified mechanisms by which function is specifically influenced by rate irregularity in the failing ventricle.
Methods
Study group
Nine patients (all males, age 65±11 years) with chronic AFib and systolic dysfunction were studied. All were scheduled for pacemaker implantation for treatment of bradycardia with either a single RV lead or biventricular device. Clinical and echocardiographic features are summarized in Table 1. Patients were all on stable medical therapy for chronic heart failure consisting of a diuretic (n=8), digoxin (n=8), a β-blocker (n=9), angiotensin converting enzyme or receptor blocker (n=9), aldosterone receptor blocker (n=3), nitrates or other vasodilator (n=3). Seven had chronic left bundle branch block patterns on the surface ECG, one had bi-fascicular block and one a normal QRS. Six patients had undergone atrioventricular nodal ablation. The remainder had advanced nodal block requiring prior pacemaker implantation and were paced 100% of the time. The protocol complied with the Declaration of Helsinki and was approved by Johns Hopkins Joint Committee on Clinical Investigation. All patients provided signed informed consent.
Protocol
Patients were sedated with midazolam and fentanyl during the study. Multi-polar pacing electrode catheters were positioned in the RV apex and a lateral coronary vein. A dual-sensor pressure–volume catheter (Millar 550–768, Millar Instruments, Houston, TX, USA) was advanced through a femoral arterial sheath and positioned in the left ventricle to lie along the longitudinal axis. All pacing leads and pressure–volume sensors were linked to an external microprocessor-based stimulation system with data acquisition capability (FLEXTIM II, Guidant Inc., St Paul, MN, USA). The computer generated an AFib sequence with RR intervals following a Poisson distribution characteristic of the natural ventricular response to AFib.13 The sequence mean rate (80 or 120 min−1) was paired with regular pacing at the identical rate (Figure 1). All pacing was applied using a biventricular lead approach (RV apex, LV lateral wall) to minimize the impact of pacing-induced dys-synchrony. Each pacing sequence spanned 160 beats (2 min at 80 min−1, 1.3 min at 120 min−1). The order of the pacing sequences was randomized to eliminate time-dependent effects. Data for each condition and mean rate were recorded twice and the results combined for each respective protocol.
Data analysis
Data were recorded by FLEXSTIM II at a sampling frequency of 1000 Hz and filtered with a 0.5 Hz high-pass filter to remove the influence of respiration. Data were then edited to remove non-captured and ectopic beats and the beat just before and after ectopic events. Peak systolic and end-diastolic pressure were determined from the LV micromanometer, the latter defined as the pressure when dP/dt reached 10% of dP/dtmax. We also normalized dP/dtmax to the instantaneous pressure (dP/dtmax/IP) and end-diastolic volume (dP/dtmax/EDV) to reduce load sensitivity. Central arterial systolic, diastolic, and digital mean pressure were obtained from the aortic micromanometer data. Calculations were performed beat-by-beat, and results averaged for each protocol and compared by paired Student's t-test. The impact of mean heart rate was tested by comparing the differences between irregular vs. regular pacing data at 80 vs. 120 b.p.m.−1 mean rate. Significance level (two-sided) was adjusted by Bonferoni correction to P<0.018 to account for the three paired comparisons with each parameter. Sample size was estimated from prior data14 indicating that a difference of >6% in key haemodynamic variables could be discerned with adequate power at n=10. We stopped enrolment at n=9 when our endpoints were clearly achieved.
This model is widely used for such force–interval relation analysis.15 The pRR/ppRR ratio was used as it normalized the RR interval of the beat just prior to the haemodynamic response to the beat preceding that. This determined whether the test beat RR interval (pRR) was shorter (pre-excitation) or longer (potentiated) than the preceding one (ppRR).
Results
All subjects completed the study protocol. The volume catheter signal was uninterpretable in three subjects due to low signal–noise ratio from marked chamber dilation and reduced ejection. This was identified at the time of study and volume data from these subjects were not analysed.
Impact of heart rate irregularity on haemodynamics varies with mean rate
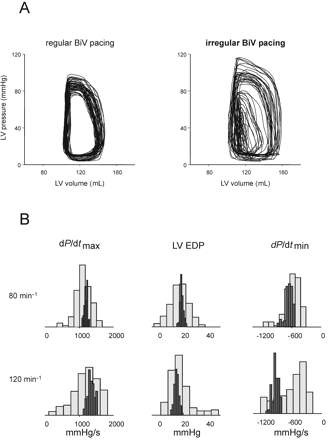
Figure 2A displays example pressure–volume loops recorded during a period of either steady state or irregular rate pacing. Histograms showing the distribution of derived chamber function parameters [dP/dtmax, dP/dtmin, and LV end-diastolic pressure (LVEDP)] are displayed in Figure 2B. Function parameters followed Gaussian distributions and were broader with AFib-type stimulation as expected. Summary data based on these distributions are provided in Table 2.
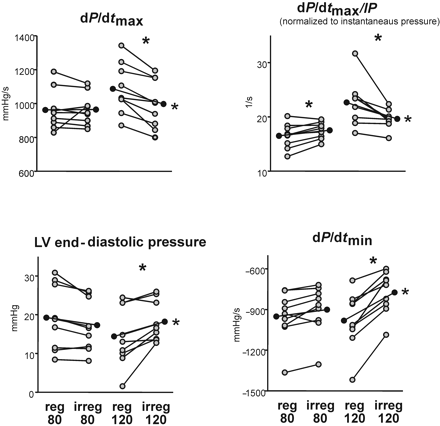
At 80 min−1 mean rate, dP/dtmax did not change and dP/dtmax/instantaneous pressure (IP) increased slightly (+6.3%, P=0.004), while dP/dtmax/LV end-diastolic volume (EDV) was not altered (P=0.7) comparing irregular vs. steady-rate biventricular pacing. In contrast, contractility was depressed by irregular vs. regular pacing at the faster (120 min−1) mean rate (dP/dtmax: −8.2%, P<0.001; dP/dtmax/IP: −12%, P=0.007; dP/dtmax/EDV: −5.5%, P=0.1) (Figure 3). Diastolic function indexed by LVEDP and dP/dtmin were also unaffected by rate irregularity at the normal rates, but increased by 26 and 21%, respectively (both P≤0.001) with irregular pacing at the faster rate. Systolic blood pressure was unchanged by rhythm regularity (at either mean heart rate), whereas mean and pulse pressure declined slightly with irregular stimulation at both rates, principally due to a greater incidence of non-ejecting beats (5.7% of the beats at 80 min−1; and 14.3% at 120 min−1). Excluding non-ejecting beats eliminated this disparity in pulse pressure. Cardiac stroke volume and end-systolic and end-diastolic volumes were similar between regular and irregular rate pacing at both mean rates. Paired analysis confirmed a significant impact of mean heart rate on the haemodynamic influence of rate irregularity for dP/dtmax, dP/dtmax/IP, dP/dtmax/EDV, dP/dtmin and LVEDP, whereas the rate interaction on other parameters was not significant (Table 2).
Determinants of ventricular performance during irregular rhythm
The dP/dtmax ratio positively correlated with the length of the preceding RR interval (pRR) at both mean heart rates (Spearman's r=0.41 and r=0.68, both P<0.001), and correlated more strongly with the pRR/ppRR ratio (r=0.69 and r=0.74, both P<0.001). At the faster mean rate, the relation between contractility and pRR/ppRR ratio was steeper, indicating enhanced force–frequency dependence (Figure 4). Similarly, dP/dtmin negatively correlated with pRR and pRR/ppRR ratios (r=−0.30 for each at both mean rates, P<0.001). Enhanced contractility with a faster mean rate (positive force–frequency response) was present with regular but not with irregular pacing (P=0.006).
Systolic function also depends upon net filling, yet the relation between LVEDP and dP/dtmax was very weak at the 80 min−1 mean rate (r=0.23, P<0.001) and non-significant at 120 min−1. As shown in Figure 4, the dependence of LVEDV on the pRR/ppRR ratio was considerably less than on contractility. Furthermore, increasing the mean pacing rate influences the dependence of contractility but not preload on pRR/ppRR.
Discussion
This study demonstrates that rate regularization in patients with heart failure and AFib improves systolic and diastolic function when mean heart rate is elevated, but has negligible effects when the rate is in the normal range. We found that contractile function and relaxation, rather than net chamber filling, are more potently influenced by rate irregularity. These data suggest that patients with heart failure and AFib, but adequate mean rate control, are unlikely to benefit further from rate regularization. Targeted use of rate regularization at higher frequencies—perhaps coupled to rate-triggered algorithms—may be beneficial.
Several prior studies in both animals and humans have assessed the effects of heart rate irregularity on cardiac function. In anaesthetized dogs, irregular LV apex pacing lowered cardiac output by 9% and produced mitral regurgitation when compared with regular pacing.1 In patients with AFib but generally preserved systolic function, irregular RV pacing resulted in 15% reduction of cardiac output and increased mean pulmonary wedge pressure when compared with regular RV pacing at the identical mean rate.2 This was also shown by Daoud et al.3 who found a 12% reduction in cardiac output at mean rates of either 80 or 120 min−1.
While these data would seem to counter the present findings, there are important differences that may explain the discrepancy. First, we studied responses in patients with markedly depressed cardiac function. Such patients have reduced pre-load sensitivity to varying cycle length due to blunting of the Frank–Starling relation, and this was confirmed by the relationship between pRR/ppRR and LV-EDV in the current data. Secondly, we utilized biventricular stimulation as opposed to single-site (RV or LV) pacing. Both of the latter modes generate contractile dys-synchrony, and the extent of dys-synchrony and its impact on mitral regurgitation may itself vary and worsen with irregular cycle lengths. Third, we employed an analysis that was more comprehensive and cardiac specific (less load dependent).
Our finding that rate irregularity had a significant adverse impact on systolic function at the faster rate is supported by recent experimental data in a canine model of AFib in which haemodynamics were assessed invasively at different mean heart rates. The differences between AFib and regular pacing rate were less at slow mean rates,16–18 although these studies found the heart rate dependence more pronounced for diastolic parameters (particularly dP/dtmin) than for systolic function.
There are several potential mechanisms for the adverse interaction of irregularity and mean heart rate. One is that the normal upward shift of the force–interval relation, which occurs with faster mean heart rate, is blunted in the failing heart due to depression of calcium handling.8 We observed a significantly higher increase of contractility (dP/dtmax/IP) with faster heart rate during regular than during irregular pacing. The pRR/ppRR–dP/dtmax relation plateau rose only slightly, with decline in function at shorter intervals matched by potentiation at longer ones. In effect, the spread of contractile function with irregular intervals effectively removed any net gain associated with mean heart rate itself. In contrast, the impact of RR interval on pre-load was minimal at either rate, which is consistent with a flat Frank–Starling curve with heart failure. Thus, the primary factor was interval dependence of cardiac function, not loading. Finally, a faster mean frequency might influence basal function by worsening the balance of coronary supply and demand. Nearly 15% of beats at the faster mean rate failed to open the aortic valve, which could affect net coronary perfusion. This hypothesis is further supported by recent data showing that diastolic coronary flow does not adequately rise during atrial fibrillation.19
Our study has potential clinical implications. Since heart rate irregularity itself does not appear to be detrimental to failing hearts when the average mean is normal, rate control rather than regularization would seem adequate for such patients. On the other hand, detrimental consequences of rate irregularity occur at faster rates, so that rate regularization could be reserved for this condition. Permanent rate regularization can be achieved by ablation of the atrioventricular node and subsequent pacing, preferably of both ventricles in order to avoid iatrogenic dys-synchrony-induced dysfunction from RV pacing.4 Alternatively, RR interval variance may be reduced by rate regularization algorithms recently implemented on pacemakers and cardiac resynchronization devices. These modes eliminate long pauses, as well as short intervals, by prolonging atrioventricular node refractoriness,20 but may accelerate overall ventricular rate.21 Long-term pacing at higher rates may obviously cancel out potential benefits of regularity. Based on our results, the effectiveness of such rate regularization strategies may be enhanced when activated only at mean heart rates above a given limit (i.e. 120 min−1). Time-limited regularization therapy may be particularly helpful for example as a device-based approach for management of paroxysmal atrial fibrillation. This applies to subjects who do not require chronic resynchronization therapy, as such patients would need biventricular pacing delivered continuously at all rates to offset underlying dys-synchrony.
In conclusion, we have directly tested the functional impact of rate regularization in heart failure patients with AFib, and have shown benefits over irregular rhythm only when the mean rate is high. This finding suggests that overall rate control is most important in these patients. Rate regularization may be used selectively in a targeted subset of patients, and modified pacemaker algorithms should apply rate regularization only when the effects are most likely to improve cardiac performance.
Acknowledgements
This study was funded by R01 AG018324 (D.A.K.) and a grant from Guidant, Inc. (R.B., D.A.K.). D.A.K. and R.B. serve as consultants to Guidant, Inc. A.K. and J.M.P. are Guidant, Inc. employees. The other authors have no relationship to disclose.
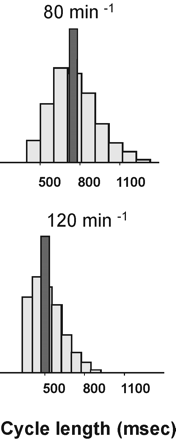
Figure 1 Histogram of pacing cycle lengths used during irregular pacing to simulate atrial fibrillation (plotted in grey) and during regular pacing (plotted in black) at the mean rate of 80 min−1 (A) and of 120 min−1 (B).
Figure 2 (A) An example of pressure–volume (pv) loops recorded during a period of either steady state or irregular rate biventricular (BiV) pacing. There was marked variability of systolic pressure and contractility with simulated AFib. End-diastolic volume (rightward points of PV loops) was relatively less changed in most beats, while clearly reduced in some particularly premature ones. Steady state loops show far less but still some variability over the same 2 min time interval. (B) Individual example of haemodynamic data presented as histograms of beat counts. Upper row shows data recorded during regular (plotted in black) and irregular (plotted in grey) pacing at the mean rate of 80 min−1, the lower row shows the data at the rate of 120 min−1.
Figure 3 The effect of irregularity on parameters of systolic and diastolic function of the left ventricle in all studied subjects (n=9). Grey dots represent individual mean value per pacing protocol. Black dots are group means. *P<0.018 for irregular pacing vs. regular pacing at the same rate. **P<0.018 for Δ(reg80–irreg80) vs. Δ(reg120–irreg120).
Figure 4 The relationship between the ratio of preceding/pre-preceding RR intervals (pRR/ppRR), contractility (dP/dtmax) and pre-load (end-diastolic volume) during irregular pacing at mean rate 80 min−1 (filled circles) and at mean rate 120 (open circles). Dots represent bin-averaged data from all subjects. The histograms of EDV and dP/dtmax values are given to the right of graph. Black lines are the distributions during irregular pacing at a mean of 80 min−1, grey lines are the distributions during irregular pacing at a mean of 120 min−1. The equation fitted is y=C*[1–e−(RRn−to)/τ].
Clinical characteristics and echocardiography of study population (n=9)
| Aetiology: CAD/DCM | 5/4 |
| NYHA class | 2.6 (1–4) |
| MLHFQ total score | 46.5 (14–82) |
| QRS duration, ms | 152 (84–244) |
| Left atrium, mm | 56 (48–67) |
| Interventricular septum, mm | 12 (6.8–15) |
| Posterior wall, mm | 12 (8.2–15) |
| End-diastolic volume, mL | 201 (338–145) |
| Ejection fraction, % | 24 (14.5–30) |
| Mitral regurgitation grade: (scale 1–4) | 1.8 (0–4) |
| Aetiology: CAD/DCM | 5/4 |
| NYHA class | 2.6 (1–4) |
| MLHFQ total score | 46.5 (14–82) |
| QRS duration, ms | 152 (84–244) |
| Left atrium, mm | 56 (48–67) |
| Interventricular septum, mm | 12 (6.8–15) |
| Posterior wall, mm | 12 (8.2–15) |
| End-diastolic volume, mL | 201 (338–145) |
| Ejection fraction, % | 24 (14.5–30) |
| Mitral regurgitation grade: (scale 1–4) | 1.8 (0–4) |
Values represent means and ranges.
CAD, coronary artery disease; DCM, dilated cardiomyopathy; NYHA, New York Heart Association; MLHFQ, Minnesota Living with Heart Failure Questionnaire.
Clinical characteristics and echocardiography of study population (n=9)
| Aetiology: CAD/DCM | 5/4 |
| NYHA class | 2.6 (1–4) |
| MLHFQ total score | 46.5 (14–82) |
| QRS duration, ms | 152 (84–244) |
| Left atrium, mm | 56 (48–67) |
| Interventricular septum, mm | 12 (6.8–15) |
| Posterior wall, mm | 12 (8.2–15) |
| End-diastolic volume, mL | 201 (338–145) |
| Ejection fraction, % | 24 (14.5–30) |
| Mitral regurgitation grade: (scale 1–4) | 1.8 (0–4) |
| Aetiology: CAD/DCM | 5/4 |
| NYHA class | 2.6 (1–4) |
| MLHFQ total score | 46.5 (14–82) |
| QRS duration, ms | 152 (84–244) |
| Left atrium, mm | 56 (48–67) |
| Interventricular septum, mm | 12 (6.8–15) |
| Posterior wall, mm | 12 (8.2–15) |
| End-diastolic volume, mL | 201 (338–145) |
| Ejection fraction, % | 24 (14.5–30) |
| Mitral regurgitation grade: (scale 1–4) | 1.8 (0–4) |
Values represent means and ranges.
CAD, coronary artery disease; DCM, dilated cardiomyopathy; NYHA, New York Heart Association; MLHFQ, Minnesota Living with Heart Failure Questionnaire.
Data from all subjects during regular and irregular pacing at mean heart rates of 80 and 120 b.p.m.
| . | 80 b.p.m. . | 120 b.p.m. . | Δ80 vs. Δ120b . | ||||
|---|---|---|---|---|---|---|---|
| . | Regular . | Irregular . | R vs. Ia . | Regular . | Irregular . | R vs. Ia . | . |
| LV dP/dtmax, mmHg/s | 962±117 | 964±93 | 0.91 | 1087±150 | 997±144 | <0.001 | 0.002 |
| LV dP/dtmax/IP, s−1 | 16.5±2.3 | 17.5±1.5 | 0.004 | 22.6±4.2 | 19.6±1.7 | 0.007 | 0.005 |
| LV dP/dtmax/EDV, mmHg/mL s) | 4.43±1.96 | 4.47±1.74 | 0.73 | 5.57±2.26 | 5.17±2.04 | 0.10 | 0.014 |
| LV dP/dtmin, mmHg/s | −952±184 | −901±179 | 0.02 | −982±211 | −774±155 | <0.001 | <0.001 |
| MAP, mmHg | 83±11 | 81±12 | 0.008 | 82±13 | 80±13 | <0.001 | 0.61 |
| SAP, mmHg | 104±9 | 102±10 | 0.05 | 97±12 | 97±13 | 0.78 | 0.81 |
| PP, mmHg | 37±10 | 34±7 | 0.002 | 26±6.9 | 23±5 | 0.002 | 0.30 |
| LVEDP, mmHg | 19.2±8.3 | 17.3±6.7 | 0.02 | 14.4±7.6 | 18.2±5.2 | 0.007 | <0.001 |
| EDV, mLc | 238±63 | 226±55 | 0.03 | 208±56 | 199±52 | 0.06 | 0.30 |
| ESV, mLc | 167±40 | 160±38 | 0.09 | 155±38 | 145±49 | 0.18 | 0.50 |
| SV, mLc | 71±39 | 66±38 | 0.02 | 53±29 | 55±37 | 0.65 | 0.25 |
| . | 80 b.p.m. . | 120 b.p.m. . | Δ80 vs. Δ120b . | ||||
|---|---|---|---|---|---|---|---|
| . | Regular . | Irregular . | R vs. Ia . | Regular . | Irregular . | R vs. Ia . | . |
| LV dP/dtmax, mmHg/s | 962±117 | 964±93 | 0.91 | 1087±150 | 997±144 | <0.001 | 0.002 |
| LV dP/dtmax/IP, s−1 | 16.5±2.3 | 17.5±1.5 | 0.004 | 22.6±4.2 | 19.6±1.7 | 0.007 | 0.005 |
| LV dP/dtmax/EDV, mmHg/mL s) | 4.43±1.96 | 4.47±1.74 | 0.73 | 5.57±2.26 | 5.17±2.04 | 0.10 | 0.014 |
| LV dP/dtmin, mmHg/s | −952±184 | −901±179 | 0.02 | −982±211 | −774±155 | <0.001 | <0.001 |
| MAP, mmHg | 83±11 | 81±12 | 0.008 | 82±13 | 80±13 | <0.001 | 0.61 |
| SAP, mmHg | 104±9 | 102±10 | 0.05 | 97±12 | 97±13 | 0.78 | 0.81 |
| PP, mmHg | 37±10 | 34±7 | 0.002 | 26±6.9 | 23±5 | 0.002 | 0.30 |
| LVEDP, mmHg | 19.2±8.3 | 17.3±6.7 | 0.02 | 14.4±7.6 | 18.2±5.2 | 0.007 | <0.001 |
| EDV, mLc | 238±63 | 226±55 | 0.03 | 208±56 | 199±52 | 0.06 | 0.30 |
| ESV, mLc | 167±40 | 160±38 | 0.09 | 155±38 | 145±49 | 0.18 | 0.50 |
| SV, mLc | 71±39 | 66±38 | 0.02 | 53±29 | 55±37 | 0.65 | 0.25 |
Paired t-tests were used, P<0.018 considered as significant.
aR vs. I column shows P values for comparison of regular pacing and irregular pacing at the same rate.
bΔ80 vs. Δ120 column shows P values for comparison of regular–irregular differences at both mean pacing frequencies.
cIn patients with interpretable volume signal.
MAP, mean arterial blood pressure; SAP, systolic arterial blood pressure; PP, arterial pulse pressure; ESV, LV end-systolic volume; SV, stroke volume.
Data from all subjects during regular and irregular pacing at mean heart rates of 80 and 120 b.p.m.
| . | 80 b.p.m. . | 120 b.p.m. . | Δ80 vs. Δ120b . | ||||
|---|---|---|---|---|---|---|---|
| . | Regular . | Irregular . | R vs. Ia . | Regular . | Irregular . | R vs. Ia . | . |
| LV dP/dtmax, mmHg/s | 962±117 | 964±93 | 0.91 | 1087±150 | 997±144 | <0.001 | 0.002 |
| LV dP/dtmax/IP, s−1 | 16.5±2.3 | 17.5±1.5 | 0.004 | 22.6±4.2 | 19.6±1.7 | 0.007 | 0.005 |
| LV dP/dtmax/EDV, mmHg/mL s) | 4.43±1.96 | 4.47±1.74 | 0.73 | 5.57±2.26 | 5.17±2.04 | 0.10 | 0.014 |
| LV dP/dtmin, mmHg/s | −952±184 | −901±179 | 0.02 | −982±211 | −774±155 | <0.001 | <0.001 |
| MAP, mmHg | 83±11 | 81±12 | 0.008 | 82±13 | 80±13 | <0.001 | 0.61 |
| SAP, mmHg | 104±9 | 102±10 | 0.05 | 97±12 | 97±13 | 0.78 | 0.81 |
| PP, mmHg | 37±10 | 34±7 | 0.002 | 26±6.9 | 23±5 | 0.002 | 0.30 |
| LVEDP, mmHg | 19.2±8.3 | 17.3±6.7 | 0.02 | 14.4±7.6 | 18.2±5.2 | 0.007 | <0.001 |
| EDV, mLc | 238±63 | 226±55 | 0.03 | 208±56 | 199±52 | 0.06 | 0.30 |
| ESV, mLc | 167±40 | 160±38 | 0.09 | 155±38 | 145±49 | 0.18 | 0.50 |
| SV, mLc | 71±39 | 66±38 | 0.02 | 53±29 | 55±37 | 0.65 | 0.25 |
| . | 80 b.p.m. . | 120 b.p.m. . | Δ80 vs. Δ120b . | ||||
|---|---|---|---|---|---|---|---|
| . | Regular . | Irregular . | R vs. Ia . | Regular . | Irregular . | R vs. Ia . | . |
| LV dP/dtmax, mmHg/s | 962±117 | 964±93 | 0.91 | 1087±150 | 997±144 | <0.001 | 0.002 |
| LV dP/dtmax/IP, s−1 | 16.5±2.3 | 17.5±1.5 | 0.004 | 22.6±4.2 | 19.6±1.7 | 0.007 | 0.005 |
| LV dP/dtmax/EDV, mmHg/mL s) | 4.43±1.96 | 4.47±1.74 | 0.73 | 5.57±2.26 | 5.17±2.04 | 0.10 | 0.014 |
| LV dP/dtmin, mmHg/s | −952±184 | −901±179 | 0.02 | −982±211 | −774±155 | <0.001 | <0.001 |
| MAP, mmHg | 83±11 | 81±12 | 0.008 | 82±13 | 80±13 | <0.001 | 0.61 |
| SAP, mmHg | 104±9 | 102±10 | 0.05 | 97±12 | 97±13 | 0.78 | 0.81 |
| PP, mmHg | 37±10 | 34±7 | 0.002 | 26±6.9 | 23±5 | 0.002 | 0.30 |
| LVEDP, mmHg | 19.2±8.3 | 17.3±6.7 | 0.02 | 14.4±7.6 | 18.2±5.2 | 0.007 | <0.001 |
| EDV, mLc | 238±63 | 226±55 | 0.03 | 208±56 | 199±52 | 0.06 | 0.30 |
| ESV, mLc | 167±40 | 160±38 | 0.09 | 155±38 | 145±49 | 0.18 | 0.50 |
| SV, mLc | 71±39 | 66±38 | 0.02 | 53±29 | 55±37 | 0.65 | 0.25 |
Paired t-tests were used, P<0.018 considered as significant.
aR vs. I column shows P values for comparison of regular pacing and irregular pacing at the same rate.
bΔ80 vs. Δ120 column shows P values for comparison of regular–irregular differences at both mean pacing frequencies.
cIn patients with interpretable volume signal.
MAP, mean arterial blood pressure; SAP, systolic arterial blood pressure; PP, arterial pulse pressure; ESV, LV end-systolic volume; SV, stroke volume.
References
Naito M, David D, Michelson EL et al. The hemodynamic consequences of cardiac arrhythmias: evaluation of the relative roles of abnormal atrioventricular sequencing, irregularity of ventricular rhythm and atrial fibrillation in a canine model.
Clark DM, Plumb VJ, Epstein AE et al. Hemodynamic effects of an irregular sequence of ventricular cycle lengths during atrial fibrillation.
Daoud EG, Weiss R, Bahu M et al. Effect of an irregular ventricular rhythm on cardiac output.
Leclercq C, Kass DA. Retiming the failing heart: principles and current clinical status of cardiac resynchronization.
Wilkoff BL, The DT, I. The Dual Chamber and VVI Implantable Defibrillator (DAVID) trial: rationale, design, results, clinical implications and lessons for future trials.
Leclercq C, Faris O, Tunin R et al. Systolic improvement and mechanical resynchronization does not require electrical synchrony in the dilated failing heart with left bundle-branch block.
Hasenfuss G, Pieske B. Calcium cycling in congestive heart failure.
Mulieri LA, Hasenfuss G, Leavitt B et al. Altered myocardial force-frequency relation in human heart failure.
Gosselink AT, Blanksma PK, Crijns HJ et al. Left ventricular beat-to-beat performance in atrial fibrillation: contribution of Frank–Starling mechanism after short rather than long RR intervals.
Suzuki S, Araki J, Morita T et al. Ventricular contractility in atrial fibrillation is predictable by mechanical restitution and potentiation.
Hardman SM, Noble MI, Seed WA. Postextrasystolic potentiation and its contribution to the beat-to-beat variation of the pulse during atrial fibrillation.
del Monte F, Hajjar RJ. Targeting calcium cycling proteins in heart failure through gene transfer.
Cohen RJ, Berger RD, Dushane TE. A quantitative model for the ventricular response during atrial fibrillation.
Kass DA, Chen CH, Curry C et al. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay.
Liu CP, Ting CT, Lawrence W et al. Diminished contractile response to increased heart rate in intact human left ventricular hypertrophy. Systolic versus diastolic determinants.
Wallick DW, Zhang Y, Tabata T et al. Selective AV nodal vagal stimulation improves hemodynamics during acute atrial fibrillation in dogs.
Zhuang S, Zhang Y, Mowrey KA et al. Ventricular rate control by selective vagal stimulation is superior to rhythm regularization by atrioventricular nodal ablation and pacing during atrial fibrillation.
Popovic ZB, Mowrey KA, Zhang Y et al. Slow rate during AF improves ventricular performance by reducing sensitivity to cycle length irregularity.
Kochiadakis GE, Skalidis EI, Kaleboubas MD et al. Effect of acute atrial fibrillation on coronary circulation.
Wittkampf FH, de Jongste MJ, Lie HI et al. Effect of right ventricular pacing on ventricular rhythm during atrial fibrillation.



![Figure 4 The relationship between the ratio of preceding/pre-preceding RR intervals (pRR/ppRR), contractility (dP/dtmax) and pre-load (end-diastolic volume) during irregular pacing at mean rate 80 min−1 (filled circles) and at mean rate 120 (open circles). Dots represent bin-averaged data from all subjects. The histograms of EDV and dP/dtmax values are given to the right of graph. Black lines are the distributions during irregular pacing at a mean of 80 min−1, grey lines are the distributions during irregular pacing at a mean of 120 min−1. The equation fitted is y=C*[1–e−(RRn−to)/τ].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/eurheartj/26/7/10.1093/eurheartj/ehi066/3/m_ehi06604.gif?Expires=1716378805&Signature=A0KIt2QRFiugnCpMkVhxvxxU54yv15k5nIfM~eu6UVADXzIEs6W40oQYgIP9f7S8ls8AZaee-vjlGYzMgtoZuR-ckj4V916h4mKNShiJzozhWcZnDAAGh4fCB7F9RVyxasfft9gGyH57yp841uRK1l2TcrHuPDE6AQ9hoSvAYIdlnOuI6vanNl4uKWcRL6imR~dVj8sswsJde0gS-i2AASXaEtt3WDbw9caqaZf4sbuKW~8jEFYHqeGBiNxBI7cBPcXJFJ1Lpp0z0QjKN3Y7TnnQHTvEw3-WQRRjDTg5CEPk3zRtfU5S31SEwL7rPNoxZT0nIBc9rynC-fito7sLyA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
