Abstract
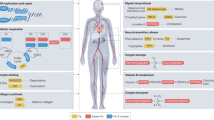
Anemia and iron deficiency are common in patients with heart failure (HF), and are associated with worse symptoms and adverse outcomes in this population. Although the two can occur together, anemia in HF is often not caused by iron deficiency, and iron deficiency can be present without causing anemia. Erythropoiesis-stimulating agents have been investigated extensively in the past few years and might be of benefit in patients with HF and anemia. However, concerns have arisen regarding the safety of erythropoiesis-stimulating agents in patients with chronic kidney disease and so the results of a large mortality trial are eagerly awaited to provide information on safety in patients with HF. Iron supplementation or replacement is a much older treatment option for patients with HF and anemia, but questions about the safety of intravenous iron, and absorption problems with oral formulations have prevented its widespread use to date. In the past few years, however, new data on the importance of iron deficiency in HF have become available, and a number of studies with intravenous iron have shown promising results. Therefore, this treatment approach is likely to become an attractive option for patients with HF and iron deficiency, both with and without anemia.
Key Points
-
Anemia and iron deficiency are both common and important in the setting of heart failure
-
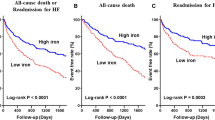
Anemia is associated with increased morbidity and mortality among patients with heart failure
-
Iron deficiency, both with and without anemia, is also associated with adverse clinical outcomes
-
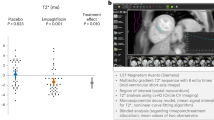
Treatment of patients with heart failure and anemia with erythropoiesis-stimulating agents could have beneficial clinical effects, but the results of a large-scale ongoing trial (RED-HF) must be awaited
-
Intravenous, but not oral, iron supplementation might be beneficial in the treatment of patients with heart failure and iron deficiency
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Anand, I. S. Anemia and chronic heart failure implications and treatment options. J. Am. Coll. Cardiol. 52, 501–511 (2008).
Groenveld, H. et al. Anemia and mortality in heart failure patients: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 52, 818–827 (2008).
Tang, Y. D. & Katz, S. D. Anemia in chronic heart failure: prevalence, etiology, clinical correlates, and treatment options. Circulation 113, 2454–2461 (2006).
Go, A. S. et al. Hemoglobin level, chronic kidney disease, and the risk of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure Outcomes and Resource Utilization (ANCHOR) Study. Circulation 113, 2713–2723 (2006).
Ezekowitz, J. A., McAlister, F. A. & Armstrong, P. W. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12,065 patients with new-onset heart failure. Circulation 107, 986–994 (2003).
Vrtovec, B. et al. Significance of anemia in patients with advanced heart failure receiving long-term mechanical circulatory support. Eur. J. Heart Fail. 11, 1000–1004 (2009).
Schroten, N. F. et al. High cumulative incidence of cancer in patients with cardio-renal-anaemia syndrome. Eur. J. Heart Fail. 12, 855–860 (2010).
Kalra, P. R. et al. Effect of anemia on exercise tolerance in chronic heart failure in men. Am. J. Cardiol. 91, 888–891 (2003).
O'Meara, E. et al. Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure: results of the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Circulation 21, 986–994 (2006).
Hogenhuis, J. et al. Anaemia and renal dysfunction are independently associated with BNP and NT-proBNP levels in patients with heart failure. Eur. J. Heart Fail. 9, 787–794 (2007).
Sharma, R. et al. Haemoglobin predicts survival in patients with chronic heart failure: a substudy of the ELITE Trial. Eur. Heart J. 25, 1021–1028 (2004).
Mozaffarian, D., Nye, R. & levy, W. C. Anemia predicts mortality in severe heart failure. The Prospective Randomized Amlodipine survival Evaluation. J. Am. Coll. Cardiol. 41, 1933–1939 (2003).
Okonko, D. O. & Anker, S. D. Anemia in chronic heart failure: pathogenetic mechanisms. J. Card. Fail. 10, S5–S9 (2004).
McClellan, W. M., Flanders, W. D., Langston, R. D., Jurkovitz, C. & Presley, R. Anemia and renal insufficiency are independent risk factors death among patients with congestive heart failure admitted to community hospitals: a population-based study. J. Am. Soc. Nephrol. 13, 1928–1936 (2002).
Anand, I. S. et al. Edema of cardiac origin. Studies of body water and sodium, renal function, hemodynamic indexes, and plasma hormones in untreated congestive heart failure. Circulation 80, 299–305 (1989).
Westenbrink, B. D. et al. Anaemia in chronic heart failure is not only related to impaired renal perfusion and blunted erythropoietin production, but to fluid retention as well. Eur. Heart J. 28, 166–171 (2007).
Androne, A. S. et al. Hemodilution is common in patients with advanced heart failure. Circulation 107, 226–229 (2003).
Van der Meer, P. et al. Levels of hematopoiesis inhibitor N-acetyl-seryl-aspartyl-lysyl-proline partially explain the occurrence of anemia in heart failure. Circulation 112, 1743–1747 (2005).
Anand, I. S. et al. Anemia and change in haemoglobin over time related to mortality and morbidity in patients with chronic heart failure: results from Val-HeFT Circulation 112, 1121–1127 (2005).
Komajda, M. et al. The impact of new onset anemia on morbidity and mortality in chronic heart failure: results from COMET. Eur. Heart J. 27, 1440–1446 (2006).
Von Haehling, S. et al. Anaemia among patients with heart failure and preserved or reduced ejection fraction: results from the SENIORS Study. Eur. J. Heart Fail. 13, 656–663 (2011).
Weiss, G. & Goodnough, L. T. Anemia of chronic disease. N. Engl. J. Med. 352, 1011–1023 (2005).
Westenbrink, B. D. et al. Bone marrow dysfunction in chronic heart failure patients. Eur. J. Heart Fail. 12, 676–684 (2010).
Kissel, C. K. et al. Selective functional exhaustion of haematopoietic progenitor cells in the bone marrow of patients with postinfarction heart failure. J. Am. Coll. Cardiol. 49, 2341–2349 (2007).
Okonko, D. O. et al. Association of deranged adrenal steroid metabolism with anemia in chronic heart failure. Am. J. Cardiol. 96, 101–103 (2005).
Van der Meer, P. et al. Adequacy of endogenous erythropoietin levels and mortality in anemic heart failure patients. Eur. Heart J. 29, 1510–1515 (2008).
Opasich, C. et al. Blunted erythropoietin production and defective iron supply for erythropoiesis as major causes of anemia in patients with chronic heart failure. Eur. Heart J. 26, 2232–2237 (2005).
Witte, K. K. et al. Are hematinic deficiencies the cause of anemia in chronic heart failure? Am. Heart J. 147, 924–930 (2004).
Wong, L. S. et al. Anaemia is associated with shorter telomere length in patients with chronic heart failure. Eur. J. Heart Fail. 12, 348–353 (2010).
de Silva, R. et al. Anemia, renal dysfunction, and their interaction in patients with chronic heart failure. Am. J. Cardiol. 98, 391–398 (2006).
Nanas, J. N. et al. Etiology of anemia in patients with advanced heart failure. J. Am. Coll. Cardiol. 48, 2485–2489 (2006).
Anker, S. D. et al. Rationale and design of Ferinject Assessment in patients with IRon deficiency and chronic Heart Failure (FAIR-HF) study: a randomized, placebo-controlled study of intravenous iron supplementation in patients with and without anaemia. Eur. J. Heart Fail. 11, 1084–1091 (2009).
Anker, S. D. et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N. Engl. J. Med. 361, 2436–2448 (2009).
González-Costello, J. & Comín-Colet, J. Iron deficiency and anemia in heart failure: understanding the FAIR-HF trial. Eur. J. Heart Fail. 12, 1159–1162 (2010).
Jankowska, E. A. et al. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur. Heart J. 31, 1872–1880 (2010).
Cavill, I. Intravenous iron as adjuvant therapy: a two-edged sword? Nephrol. Dial. Transplant. 18(Suppl 8), viii24–viii28 (2003).
Silverberg, D. S, Iaina, A., Schwartz, D. & Wexler, D. Intravenous iron in heart failure: beyond targeting anemia. Curr. Heart Fail. Rep. 8, 14–21 (2011).
Van der Meer, P. et al. Prognostic value of plasma erythropoietin on mortality in patients with chronic heart failure. J. Am. Coll. Cardiol. 44, 63–67 (2004).
Belonje, A. M., Voors, A. A., Van der Meer, P., Van Gilst. W. H. & Van Veldhuisen, D. J. Endogenous erythropoietin and outcome in heart failure. Circulation 121, 245–251 (2010).
Macdougall, I. C. & Eckardt, K. U. Novel strategies for stimulating erythropoiesis and potential new treatments for anaemia. Lancet 368, 947–953 (2006).
Silverberg, D. S. et al. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J. Am. Coll. Cardiol. 35, 1737–1744 (2000).
Silverberg, D. S. et al. The effect of correction of mild anemia in severe, resistant congestive heart failure using subcutaneous erythropoietin and intravenous iron: a randomized controlled study. J. Am. Coll. Cardiol. 37, 1775–1780 (2001).
Mancini, D. M. et al. Effect of erythropoietin on exercise capacity in patients with moderate to severe heart chronic failure. Circulation 107, 294–299 (2003).
Palazzuoli, A. et al. Erythropoietin improves anemia exercise tolerance and renal function and reduces B-type natriuretic peptide and hospitalization in patients with heart failure and anemia. Am. Heart J. 152, 1096–1015 (2006).
Parassis, J. T. et al. Effects of darbepoetin alfa on right and left ventricular systolic and diastolic function in anemic patients with chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. Heart J. 155, 751–757 (2008).
Ponikowski, P. et al. Effect of darbepoetin alfa on exercise tolerance in anemic patients with symptomatic chronic heart failure: a randomized, double-blind, placebo-controlled trial. J. Am. Coll. Cardiol. 49, 753–762 (2007).
Van Veldhuisen, D. J. et al. Randomized, double-blind, placebo-controlled study to evaluate the effect of two dosing regimens of darbepoetin alfa in patients with heart failure and anaemia. Eur. Heart J. 18, 2208–2216 (2007).
Ghali, J. K. et al. Randomized double-blind trial of darbepoetin alfa in patients with symptomatic heart failure and anemia. Circulation 117, 526–535 (2008).
Van der Meer, P., Groenveld, H. F., Januzzi, J. L. & Van Veldhuisen, D. J. Erythropoietin treatment in patients with chronic heart failure: a meta-analysis. Heart 95, 1309–1314 (2009).
McMurray, J. J. et al. Design of the Reduction of Events with darbepoetin alfa in Heart Failure (RED-HF): a phase III, anaemia correction, morbidity-mortality trial. Eur. J. Heart Fail. 11, 795–801 (2009).
Singh, A. K. et al. Correction of anemia with epoetin alfa in chronic kidney disease. N. Engl. J. Med. 355, 2085–2098 (2006).
Drueke, T. B. et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N. Engl. J. Med. 355, 2071–2084 (2006).
Van Veldhuisen, D. J. & McMurray, J. J. Are erythropoietin stimulating proteins safe and efficacious in heart failure? Why we need an adequately powered randomised outcome trial. Eur. J. Heart Fail. 9, 110–112 (2007).
Pfeffer, M. A. et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N. Engl. J. Med. 361, 2019–2032 (2009).
Desai, A., Lewis, E., Solomon, S., McMurray, J. J. & Pfeffer, M. Impact of erythropoiesis-stimulating agents on morbidity and mortality in patients with heart failure: an updated, post-TREAT meta-analysis. Eur. J. Heart Fail. 12, 936–942 (2010).
Szczech, L. A. et al. A secondary analysis of the CHOIR trial shows that comorbid conditions differentially affect outcomes during anemia treatment. Kidney Int. 77, 239–246 (2010).
Westenbrink, B. D. et al. Erythropoietin improves cardiac function through endothelial progenitor cell and vascular endothelial growth factor mediated neovascularization. Eur. Heart J. 28, 2018–2027 (2007).
De Boer, R. A., Pinto, Y. M., Van Veldhuisen, D. J. The imbalance between oxygen demand and supply as a potential mechanism in the pathophysiology of heart failure: the role of microvascular growth and abnormalities. Mircocirculation 10, 113–126 (2003).
Lipsic, E. et al. Protective effects of erythropoietin in cardiac ischemia: from bench to bedside. J. Am. Coll. Cardiol. 48, 2161–2167 (2006).
Voors, A. A. et al. A single dose of erythropoietin in ST-elevation myocardial infarction. Eur. Heart J. 31, 2593–2600 (2010).
Binbrek, A. S., Rao, N. S., Al Khaja, N., Asseqqaf, J. & Sobel, B. E. Erythropoietin to augment myocardial salvage induced by coronary thrombolysis in patients with ST-elevation acute myocardial infarction. Am. J. Cardiol. 104, 1035–1040 (2009).
Ehrenreich, H. et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol. Med. 8, 495–505 (2002).
Ehrenreich, H. et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke 40, e647–e656 (2009).
Van der Meer, P. et al. Erythropoietin improves left ventricular function and coronary flow in an experimental model of ischemia-reperfusion injury. Eur. J. Heart Fail. 6, 853–859 (2004).
Koury, M. J. & Bondurant, M. C. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science 248, 378–381 (1990).
Lipsic, E. et al. Low-dose erythropoietin improves cardiac function in experimental heart failure without increasing haematocrit. Eur. J. Heart Fail. 10, 22–29 (2008).
Moon, C. et al. Erythropoietin, modified to not stimulate red blood cell production, retains its cardioprotective properties. J. Pharmacol. Exp. Ther. 316, 999–1005 (2006).
Ogino, A. et al. Erythropoietin receptor signalling mitigates renal dysfunction-associated heart failure by mechanisms unrelated to relief of anemia. J. Am. Coll. Cardiol. 56, 1949–1958 (2010).
Klapholz, M. et al. The safety and tolerability of darbepoetin alfa in patients with anaemia and heart failure. Eur. J. Heart Fail. 11, 1071–1077 (2009).
Kazory, A. & Ross, E. A. Anemia: the point of convergence or divergence for kidney disease and heart failure. J. Am. Coll. Cardiol. 53, 639–647 (2009).
Phrommintikul, A., Haas, S. J., Elsik, M. & Krum, H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet 369, 381–388 (2007).
Macdougall, I. C. & Cooper, A. C. Hyporesponsiveness to erythropoietic therapy due to chronic inflammation. Eur. J. Clin. Invest. 35 (Suppl 3), 32–35 (2005).
Regidor, D. L. et al. Associations between changes in haemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J. Am. Soc. Nephrol. 17, 1181–1191 (2006).
Solomon, S. D. et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N. Engl. J. Med. 363, 1146–1155 (2010).
Abraham, W. T. et al. Treatment of anemia with darbepoetin alfa in heart failure. Congest. Heart Fail. 16, 87–95 (2010).
Rossert, J., Gassmann-Mayer, C., Frei, D. & McClellan, W. Prevalence and predictors of epoetin hyporeponsiveness in chronic kidney disease patients. Nephrol. Dial. Transplant. 22, 794–800 (2007).
Van der Putten, K. et al. Hepcidin-25 is a marker of the response rather than resistance to exogenous erythropoietin in chronic kidney disease/chronic heart failure patients. Eur. J. Heart Fail. 12, 943–950 (2010).
Dallalio, G., Law, E. & Means, R. T. Jr. Hepcidin inhibits in vitro erythroid colony formation at reduced erythropoietin concentrations. Blood 107, 2702–2704 (2006).
Streja, E. et al. Erythropoietin, iron depletion, and relative thrombocytosis: a possible explanation for hemoglobin-survival paradox in hemodialysis. Am. J. Kidney Dis. 52, 727–736 (2008).
Dahl, N. V., Henry, D. H. & Coyne, D. W. Thrombosis with erythropoietic stimulating agents- Does iron deficient erythropoiesis play a role? Semin. Dial. 21, 210–211 (2008).
Fairbanks, V. & Beutler, E. in Williams Hematology 6th edn (ed Beutler, E.) 295–304, 447–440 (McGraw-Hill, New York, 2001).
Dunn, L. L., Rahmanto, Y. S. & Richardson, D. R. Iron uptake and metabolism in the new millennium. Trends Cell Biol. 17, 93–100 (2007).
Anderson, G. J. & Vulpe, C. D. Mammalian iron transport. Cell. Mol. Life Sci. 66, 3241–3261 (2009).
Cairo, G., Bernuzzi, F. & Recalcati, S. A precious metal: iron, an essential nutrient for all cells. Genes Nutr. 1, 25–39 (2006).
Andrews, N. C. Disorders of iron metabolism. N. Engl. J. Med. 341, 1986–1995 (1999).
Sutak, R., Lesuisse, E., Tachezy, J. & Richardson, D. R. Crusade for iron: iron uptake in unicellular eukaryocytes and its significance for virulence. Trends Microbiol. 16, 261–268 (2008).
Wilson, M. T. & Reeder, B. J. Oxygen-binding haem proteins. Exp. Physiol. 93, 128–132 (2008).
Zimmerman, M. B. & Hurrell, R. F. Nutritional iron deficiency. Lancet 370, 511–520 (2007).
Anker, S. D. & Sharma, R. The syndrome of cardiac cachexia. Int. J. Cardiol. 85, 51–66 (2002).
Weiss, G. Iron metabolism in the anemia of chronic disease. Biochim. Biophys. Acta 1790, 682–693 (2009).
Gisbert, J. P. & Gomollon, F. An update on iron physiology. World J. Gastroenterol. 15, 4659–4665 (2009).
Dong, F. et al. Dietary iron deficiency induce ventricular dilation, mitochondrial ultrastructural aberrations and cytochrome c release: involvement of nitric oxide synthese and protein tyrosine nitration. Clin. Sci. (Lond.) 109, 277–286 (2005).
Brownlie, T., Utermohlen, V., Hinton, P. S. & Haas, J. D. Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. Am. J. Clin. Nutr. 79, 437–443 (2004).
Haas, J. D. & Brownlie, T. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J. Nutr. 131, 676S–690S (2001).
Handelman, G. J. & Levin, N. W. Iron anemia in human biology. A review of mechanisms. Heart Fail. Rev. 13, 393–404 (2008).
Merck Research Laboratories, Merck & Co Inc. The Merck Manual of Diagnosis and Therapy 16th edn 1144 (Merck & Co Inc., Rahway, 1992).
Mann, D. L. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ. Res. 91, 988–998 (2002).
Heymans, S. et al. Inflammation as a therapeutic target in heart failure? A scientific statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 11, 119–129 (2009).
Naito, Y. et al. Impaired expression of duodenal iron transporters in Dahl salt-sensitive heart failure rats. J. Hypertens. 29, 741–748 (2011).
Merle, U., Fein, E., Gehrke, S. G., Stremmel, W. & Kulaksiz, H. The iron regulatory peptide hepcidin is expressed in the heart and regulated by hypoxia and inflammation. Endocrinology 148, 2663–2668 (2007).
Matsumoto, M. et al. Iron regulatory hormone hepcidin decreases in chronic heart failure patients with anemia. Circ. J. 74, 301–306 (2010).
Nicholas, G. et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc. Natl. Acad. Sci. U. S. A. 99, 4596–4601 (2002).
Ganz, T. & Nemeth, E. Hepcidin and disorders of iron metabolism. Annu. Rev. Med. 62, 347–360 (2011).
Nemeth, E. et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306, 2090–2093 (2004).
Sunder-Plassmann, G. & Höri, W. H. Iron metabolism and iron substitution during erythropoietin therapy. Clin. Invest. 72, S11–S15 (1994).
Macdougall, I. C. et al. A randomized controlled study of iron supplementation in patients treated with erythropoietin. Kidney Int. 50, 1694–1699 (1996).
Van Wyck, D. B., Roppolo, M., Martinez, C. O., Mazey, R. M. & McMurray, S, for the United States Iron Sucrose (Venefor) Clinical Trials Group. A randomized, controlled trial comparing IV iron sucrose to oral iron in anemic patients with nondialysis-dependent CKD. Kidney Int. 68, 2846–2856 (2005).
Besarab, A., Hörl, W. H. & Silverberg, D. Iron metabolism, iron deficiency, thrombocytosis, and the cardiorenal anemia syndrome. The Oncologist 14 (Suppl 1), 22–33 (2009).
Weinberg, E. D. The hazards of iron loading. Metallomics 2, 732–740 (2010).
Sullivan, J. L. Long-term risk of increased use of intravenous iron. Lancet 370, 481–482 (2007).
Cooper, C. E. Nitric oxide and iron proteins. Biochim. Biophys. Acta 1411, 290–309 (1999).
Comín-Colet, J. et al. A pilot evaluation of the long-term effect of combined therapy with intravenous iron sucrose and erythropoietin in elderly patients with advanced chronic heart failure and cardio-renal anemia syndrome: influence on neurohormonal activation and clinical outcomes. J. Card. Fail. 15, 727–735 (2009).
Bolger, A. P. et al. Intravenous iron alone for the treatment of anemia in patients with chronic heart failure. J. Am. Coll. Cardiol. 48, 1225–1227 (2006).
Usmanov, R. I., Zueva, E. B., Silverberg, D. S. & Shaked, M. Intravenous iron without erythropoietin for the treatment of iron deficiency anemia in patients with moderate to severe congestive heart failure and chronic kidney insufficiency. J. Nephrol. 21, 236–242 (2008).
Toblli, J. E., Lombraña, A., Duarte, P. & Di Genarro, F. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemia patients with chronic heart failure and renal insufficiency. J. Am. Coll. Cardiol. 50, 1657–1665 (2007).
Okonko, D. O. et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with chronic heart failure and iron deficiency. FERRIC-HF: a randomized, controlled, observer-blinded trial. J. Am. Coll. Cardiol. 51, 103–112 (2008).
Author information
Authors and Affiliations
Contributions
All authors contributed to discussion of content for the article. After the first version, which was written by D. J. van Veldhuisen, all authors researched data to include in the manuscript, and all wrote parts of the manuscript. All authors reviewed and edited the manuscript before submission, and revised the manuscript in response to the peer-reviewers' comments.
Corresponding author
Ethics declarations
Competing interests
D. J. van Veldhuisen, S. D. Anker and P. Ponikowski have been consultants for and received grant/research support from Amgen (manufacturer of darbepoetin alfa) and Vifor (manufacturer of ferric carboxymaltose [intravenous iron]). In addition, S. D. Anker and P. Ponikowski have received speakers bureau (honoraria) from Vifor, and P. Ponikowski has also received speakers bureau from Amgen. I. C. Macdougall has been a consultant for and received speakers bureau and grant/research support from Amgen, Ortho Biotech, Roche and Vifor. In addition, I. C. Macdougall has been a consultant for and received grant/research support from Affymax.
Rights and permissions
About this article
Cite this article
van Veldhuisen, D., Anker, S., Ponikowski, P. et al. Anemia and iron deficiency in heart failure: mechanisms and therapeutic approaches. Nat Rev Cardiol 8, 485–493 (2011). https://doi.org/10.1038/nrcardio.2011.77
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2011.77
This article is cited by
-
The treatment of chronic anemia in heart failure: a global approach
Clinical Research in Cardiology (2023)
-
Integrative analyses of biomarkers and pathways for heart failure
BMC Medical Genomics (2022)
-
Budget Impact of Intravenous Iron Therapy with Ferric Carboxymaltose in Patients with Chronic Heart Failure with Reduced Ejection Fraction (HFrEF) and Iron Deficiency in Switzerland
PharmacoEconomics - Open (2022)
-
Anemia in heart failure: still an unsolved enigma
The Egyptian Heart Journal (2021)
-
Neurohormonal activation induces intracellular iron deficiency and mitochondrial dysfunction in cardiac cells
Cell & Bioscience (2021)