Abstract
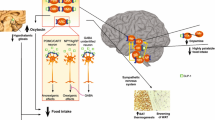
Treatment with second generation antipsychotics (SGAs), notably olanzapine and clozapine, causes severe obesity side effects. Antagonism of histamine H1 receptors has been identified as a main cause of SGA-induced obesity, but the molecular mechanisms associated with this antagonism in different stages of SGA-induced weight gain remain unclear. This review aims to explore the potential role of hypothalamic histamine H1 receptors in different stages of SGA-induced weight gain/obesity and the molecular pathways related to SGA-induced antagonism of these receptors. Initial data have demonstrated the importance of hypothalamic H1 receptors in both short- and long-term SGA-induced obesity. Blocking hypothalamic H1 receptors by SGAs activates AMP-activated protein kinase (AMPK), a well-known feeding regulator. During short-term treatment, hypothalamic H1 receptor antagonism by SGAs may activate the AMPK—carnitine palmitoyltransferase 1 signaling to rapidly increase caloric intake and result in weight gain. During long-term SGA treatment, hypothalamic H1 receptor antagonism can reduce thermogenesis, possibly by inhibiting the sympathetic outflows to the brainstem rostral raphe pallidus and rostral ventrolateral medulla, therefore decreasing brown adipose tissue thermogenesis. Additionally, blocking of hypothalamic H1 receptors by SGAs may also contribute to fat accumulation by decreasing lipolysis but increasing lipogenesis in white adipose tissue. In summary, antagonism of hypothalamic H1 receptors by SGAs may time-dependently affect the hypothalamus-brainstem circuits to cause weight gain by stimulating appetite and fat accumulation but reducing energy expenditure. The H1 receptor and its downstream signaling molecules could be valuable targets for the design of new compounds for treating SGA-induced weight gain/obesity.


Similar content being viewed by others
References
Lett TAP, Wallace TJM, Chowdhury NI, et al. Pharmacogenetics of antipsychotic-induced weight gain: review and clinical implications. Mol Psychiatry. 2012;17(3):242–66.
Parsons B, Allison DB, Loebel A, et al. Weight effects associated with antipsychotics: a comprehensive database analysis. Schizophr Res. 2009;110(1–3):103–10.
Correll CU, Lencz T, Malhotra AK. Antipsychotic drugs and obesity. Trends Mol Med. 2011;17(2):97–107.
Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123(2–3):225–33.
Roerig JL, Steffen KJ, Mitchell JE. Atypical antipsychotic-induced weight gain: insights into mechanisms of action. CNS Drugs. 2011;25(12):1035–59.
Deng C, Weston-Green K, Huang XF. The role of histaminergic H1 and H3 receptors in food intake: a mechanism for atypical antipsychotic-induced weight gain? Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(1):1–4.
Nasrallah HA. Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatr. 2008;13(1):27–35.
Matsui-Sakata A, Ohtani H, Sawada Y. Receptor occupancy-based analysis of the contributions of various receptors to antipsychotics-induced weight gain and diabetes mellitus. Drug Metab Pharmacokinet. 2005;20(5):368–78.
Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88(3):1183–241.
Brown RE, Stevens DR, Haas HL. The physiology of brain histamine. Prog Neurobiol. 2001;63(6):637–72.
Masaki T, Yoshimatsu H. The hypothalamic H1 receptor: a novel therapeutic target for disrupting diurnal feeding rhythm and obesity. Trends Pharmacol Sci. 2006;27(5):279–84.
Clineschmidt BV, Lotti VJ. Histamine: intraventricular injection suppresses ingestive behavior of the cat. Arch Int Pharmacodyn Ther. 1973;206(2):288–98.
Itowi N, Nagai K, Nakagawa H, et al. Changes in the feeding behavior of rats elicited by histamine infusion. Physiol Behav. 1988;44(2):221–6.
Lecklin A, Etu-Seppala P, Stark H, et al. Effects of intracerebroventricularly infused histamine and selective H-1, H-2 and H-3 agonists on food and water intake and urine flow in Wistar rats. Brain Res. 1998;793(1–2):279–88.
Masaki T, Yoshimatsu H, Chiba S, et al. Central infusion of histamine reduces fat accumulation and upregulates UCP family in leptin-resistant obese mice. Diabetes. 2001;50(2):376–84.
Masaki T, Chiba S, Yoshimichi G, et al. Neuronal histamine regulates food intake, adiposity, and uncoupling protein expression in agouti yellow (A(y)/a) obese mice. Endocrinology. 2003;144(6):2741–8.
Fulop AK, Foldes A, Buzas E, et al. Hyperleptinemia, visceral adiposity, and decreased glucose tolerance in mice with a targeted disruption of the histidine decarboxylase gene. Endocrinology. 2003;144(10):4306–14.
Jorgensen EA, Vogelsang TW, Knigge U, et al. Increased susceptibility to diet-induced obesity in histamine-deficient mice. Neuroendocrinology. 2006;83(5–6):289–94.
Masaki T, Chiba S, Yasuda T, et al. Involvement of hypothalamic histamine H-1 receptor in the regulation of feeding rhythm and obesity. Diabetes. 2004;53(9):2250–60.
Chervinsky P, Georgitis J, Banov C, et al. Once daily loratadine versus astemizole once daily. Ann Allergy. 1994;73(2):109–13.
Saleh JW, Yang MU, van Itallie TB, et al. Ingestive behavior and composition of weight change during cyproheptadine administration. Int J Obes. 1979;3(3):213–21.
Silverstone T, Schuyler D. The effect of cyproheptadine on hunger, calorie intake and body weight in man. Psychopharmacologia. 1975;40(4):335–40.
Ratliff JC, Barber JA, Palmese LB, et al. Association of prescription H1 antihistamine use with obesity: results from the National Health and Nutrition Examination Survey. Obesity (Silver Spring). 2010;18(12):2398–400.
Fukagawa K, Sakata T, Shiraishi T, et al. Neuronal histamine modulates feeding behavior through H1-receptor in rat hypothalamus. Am J Physiol. 1989;256(3 Pt 2):R605–11.
Sakata T, Ookuma K, Fukagawa K, et al. Blockade of the histamine H1-receptor in the rat ventromedial hypothalamus and feeding elicitation. Brain Res. 1988;441(1–2):403–7.
Ookuma K, Yoshimatsu H, Sakata T, et al. Hypothalamic sites of neuronal histamine action on food intake by rats. Brain Res. 1989;490(2):268–75.
Ookuma K, Sakata T, Fukagawa K, et al. Neuronal histamine in the hypothalamus suppresses food intake in rats. Brain Res. 1993;628(1–2):235–42.
Umehara H, Mizuguchi H, Mizukawa N, et al. Innervation of histamine neurons in the caudal part of the arcuate nucleus of hypothalamus and their activation in response to food deprivation under scheduled feeding. Methods Find Exp Clin Pharmacol. 2010;32(10):733–6.
Umehara H, Mizuguchi H, Mizukawa N, et al. Deprivation of anticipated food under scheduled feeding induces c-Fos expression in the caudal part of the arcuate nucleus of hypothalamus through histamine H1 receptors in rats: Potential involvement of E3 subgroup of histaminergic neurons in tuberomammillary nucleus. Brain Res. 2011;1387:61–70.
Gomez-Ramirez J, Ortiz J, Blanco I. Presynaptic H3 autoreceptors modulate histamine synthesis through cAMP pathway. Mol Pharmacol. 2002;61(1):239–45.
Arrang JM, Garbarg M, Schwartz JC. Autoinhibition of histamine synthesis mediated by presynaptic H3-receptors. Neuroscience. 1987;23(1):149–57.
Hancock AA, Brune ME. Assessment of pharmacology and potential anti-obesity properties of H-3 receptor antagonists/inverse agonists. Expert Opin Investig Drugs. 2005;14(3):223–41.
Passani MB, Blandina P, Torrealba F. The histamine H3 receptor and eating behavior. J Pharmacol Exp Ther. 2011;336(1):24–9.
Threlfell S, Cragg SJ, Kallo I, et al. Histamine H3 receptors inhibit serotonin release in substantia nigra pars reticulata. J Neurosci. 2004;24(40):8704–10.
Arrang JM, Drutel G, Schwartz JC. Characterization of histamine H3 receptors regulating acetylcholine release in rat entorhinal cortex. Br J Pharmacol. 1995;114(7):1518–22.
Molina-Hernandez A, Nunez A, Arias-Montano JA. Histamine H3-receptor activation inhibits dopamine synthesis in rat striatum. Neuroreport. 2000;11(1):163–6.
Hong ST, Bang S, Paik D, et al. Histamine and its receptors modulate temperature-preference behaviors in Drosophila. J Neurosci. 2006;26(27):7245–56.
Yasuda T, Masaki T, Sakata T, et al. Hypothalamic neuronal histamine regulates sympathetic nerve activity and expression of uncoupling protein 1 mRNA in brown adipose tissue in rats. Neuroscience. 2004;125(3):535–40.
Lundius EG, Sanchez-Alavez M, Ghochani Y, et al. Histamine influences body temperature by acting at H1 and H3 receptors on distinct populations of preoptic neurons. J Neurosci. 2010;30(12):4369–81.
Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci. 2011;16:74–104.
Sethi J, Sanchez-Alavez M, Tabarean IV. Loss of histaminergic modulation of thermoregulation and energy homeostasis in obese mice. Neuroscience. 2012;217:84–95.
Tabarean IV, Sanchez-Alavez M, Sethi J. Mechanism of H2 histamine receptor dependent modulation of body temperature and neuronal activity in the medial preoptic nucleus. Neuropharmacology. 2012;63(2):171–80.
Morrison SF, Madden CJ, Tupone D. Central control of brown adipose tissue thermogenesis. Front Endocrinol (Lausanne) 2012;3(5).
Chen QH, Toney GM. In vivo discharge properties of hypothalamic paraventricular nucleus neurons with axonal projections to the rostral ventrolateral medulla. J Neurophysiol. 2010;103(1):4–15.
Cham JL, Badoer E. Exposure to a hot environment can activate rostral ventrolateral medulla-projecting neurones in the hypothalamic paraventricular nucleus in conscious rats. Exp Physiol. 2008;93(1):64–74.
Madden CJ, Morrison SF. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R831–43.
Tsuda K, Yoshimatsu H, Niijima A, et al. Hypothalamic histamine neurons activate lipolysis in rat adipose tissue. Exp Biol Med (Maywood). 2002;227(3):208–13.
Bugajski J, Janusz Z. Lipolytic responses induced by intracerebroventricular administration of histamine in the rat. Agents Actions. 1981;11(1–2):147–50.
Carpene C, Morin N, Fontana E, et al. Histamine weakly stimulates lipolysis and is poorly oxidized by amine oxidases in human subcutaneous fat cells. Inflamm Res. 2001;50:S140–1.
Wang KY, Tanimoto A, Yamada S, et al. Histamine regulation in glucose and lipid metabolism via histamine receptors: model for nonalcoholic steatohepatitis in mice. Am J Pathol. 2010;177(2):713–23.
Shen J, Tanida M, Yao JF, et al. Biphasic effects of orexin-A on autonomic nerve activity and lipolysis. Neurosci Lett. 2008;444(2):166–71.
Stanley S, Pinto S, Segal J, et al. Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically. Proc Natl Acad Sci USA. 2010;107(15):7024–9.
Song CK, Schwartz GJ, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R501–11.
Coccurello R, Moles A. Potential mechanisms of atypical antipsychotic-induced metabolic derangement: clues for understanding obesity and novel drug design. Pharmacol Ther. 2010;127(3):210–51.
Haddad P. Weight change with atypical antipsychotics in the treatment of schizophrenia. J Psychopharmacol. 2005;19(6 Suppl):16–27.
Gentile S. Long-term treatment with atypical antipsychotics and the risk of weight gain: a literature analysis. Drug Saf. 2006;29(4):303–19.
Kroeze WK, Hufeisen SJ, Popadak BA, et al. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28(3):519–26.
Kim SF, Huang AS, Snowman AM, et al. Antipsychotic drug-induced weight gain mediated by histamine H-1 receptor-linked activation of hypothalamic AMP-kinase. Proc Natl Acad Sci USA. 2007;104(9):3456–9.
Vehof J, Risselada AJ, Al Hadithy AFY, et al. Association of genetic variants of the histamine H1 and muscarinic M3 receptors with BMI and HbA1c values in patients on antipsychotic medication. Psychopharmacology (Berl). 2011;216(2):257–65.
Hong CJ, Lin CH, Yu YW, et al. Genetic variant of the histamine-1 receptor (glu349asp) and body weight change during clozapine treatment. Psychiatr Genet. 2002;12(3):169–71.
Basile VS, Masellis M, McIntyre RS, et al. Genetic dissection of atypical antipsychotic-induced weight gain: novel preliminary data on the pharmacogenetic puzzle. J Clin Psychiatry. 2001;62(Suppl 23):45–66.
Han M, Deng C, Burne THJ, et al. Short- and long-term effects of antipsychotic drug treatment on weight gain and H1 receptor expression. Psychoneuroendocrinology. 2008;33(5):569–80.
Kirk SL, Glazebrook J, Grayson B, et al. Olanzapine-induced weight gain in the rat: role of 5-HT2C and histamine H1 receptors. Psychopharmacology (Berl). 2009;207(1):119–25.
Poyurovsky M, Pashinian A, Levi A, et al. The effect of betahistine, a histamine H1 receptor agonist/H3 antagonist, on olanzapine-induced weight gain in first-episode schizophrenia patients. Int Clin Psychopharmacol. 2005;20(2):101–3.
Deng C, Lian J, Pai N, et al. Reducing olanzapine-induced weight gain side-effect by betahistine: a study in the rat model. J Psychopharmacol. 2012;13:2012.
Poyurovsky M, Fuchs C, Pashinian A, et al. Reducing antipsychotic-induced weight gain in schizophrenia: a double-blind placebo-controlled study of reboxetine-betahistine combination. Psychopharmacology (Berl) 2013;226(3):615–22.
Poyurovsky M, Fuchs C, Pashinian A, et al. Attenuating effect of reboxetine on appetite and weight gain in olanzapine-treated schizophrenia patients: a double-blind placebo-controlled study. Psychopharmacology (Berl). 2007;192(3):441–8.
Lage R, Dieguez C, Vidal-Puig A, et al. AMPK: a metabolic gauge regulating whole-body energy homeostasis. Trends Mol Med. 2008;14(12):539–49.
Lim CT, Kola B, Korbonits M. AMPK as a mediator of hormonal signalling. J Mol Endocrinol. 2010;44(2):87–97.
de Morentin PBM, Gonzalez CR, Saha AK, et al. Hypothalamic AMP-activated protein kinase as a mediator of whole body energy balance. Rev Endocr Metab Disord. 2011;12(3):127–40.
Andersson U, Filipsson K, Abbott CR, et al. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279(13):12005–8.
Kola B. Role of AMP-activated protein kinase in the control of appetite. J Neuroendocrinol. 2008;20(7):942–51.
Kang JA, Lee K, Lee KM, et al. Desipramine inhibits histamine H1 receptor-induced Ca2+ signaling in rat hypothalamic cells. Plos One. 2012;7(4):e36185.
Ronnett GV, Kleman AM, Kim EK, et al. Fatty acid metabolism, the central nervous system, and feeding. Obesity. 2006;14:201–7.
Lane MD, Wolfgang M, Cha SH, et al. Regulation of food intake and energy expenditure by hypothalamic malonyl-CoA. Int J Obes (Lond). 2008;32(Suppl 4):S49–54.
Pocai A, Lam TK, Obici S, et al. Restoration of hypothalamic lipid sensing normalizes energy and glucose homeostasis in overfed rats. J Clin Invest. 2006;116(4):1081–91.
Obici S, Feng ZH, Arduini A, et al. Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat Med. 2003;9(6):756–61.
Wolfgang MJ, Lane MD. Hypothalamic malonyl-CoA and CPT1c in the treatment of obesity. FEBS J. 2011;278(4):552–8.
Gao S, Zhu GJ, Gao XF, et al. Important roles of brain-specific carnitine palmitoyltransferase and ceramide metabolism in leptin hypothalamic control of feeding. Proc Natl Acad Sci USA. 2011;108(23):9691–6.
Wolfgang MJ, Kurama T, Dai Y, et al. The brain-specific carnitine palmitoyltransferase-1c regulates energy homeostasis. Proc Natl Acad Sci USA. 2006;103(19):7282–7.
Sierra AY, Gratacos E, Carrasco P, et al. CPT1c is localized in endoplasmic reticulum of neurons and has carnitine palmitoyltransferase activity. J Biol Chem. 2008;283(11):6878–85.
Lopez M, Lage R, Saha AK, et al. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab. 2008;7(5):389–99.
Kohno D, Sone H, Tanaka S, et al. AMP-activated protein kinase activates neuropeptide Y neurons in the hypothalamic arcuate nucleus to increase food intake in rats. Neurosci Lett. 2011;499(3):194–8.
Martins PJ, Haas M, Obici S. Central nervous system delivery of the antipsychotic olanzapine induces hepatic insulin resistance. Diabetes. 2010;59(10):2418–25.
Sejima E, Yamauchi A, Nishioku T, et al. A role for hypothalamic AMP-activated protein kinase in the mediation of hyperphagia and weight gain induced by chronic treatment with olanzapine in female rats. Cell Mol Neurobiol. 2011;31(7):985–9.
Ferno J, Varela L, Skrede S, et al. Olanzapine-induced hyperphagia and weight gain associate with orexigenic hypothalamic neuropeptide signaling without concomitant AMPK phosphorylation. Plos One. 2011;6(6):e20571.
Kim MK, Kim SH, Yu HS, et al. The effect of clozapine on the AMPK-ACC-CPT1 pathway in the rat frontal cortex. Int J Neuropsychopharmacol. 2012;15(7):907–17.
Deng C, Weston-Green KL, Han M, et al. Olanzapine treatment decreases the density of muscarinic M2 receptors in the dorsal vagal complex of rats. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(4):915–20.
Weston-Green K, Huang XF, Han M, et al. The effects of antipsychotics on the density of cannabinoid receptors in the dorsal vagal complex of rats: implications for olanzapine-induced weight gain. Int J Neuropsychopharmacol. 2008;11(6):827–35.
Blevins JE, Baskin DG. Hypothalamic-brainstem circuits controlling eating. Forum Nutr. 2010;63:133–40.
Schwartz GJ. Integrative capacity of the caudal brainstem in the control of food intake. Philos Trans R Soc Lond B Biol Sci. 2006;361(1471):1275–80.
Watanabe T, Taguchi Y, Shiosaka S, et al. Distribution of the histaminergic neuron system in the central nervous system of rats; a fluorescent immunohistochemical analysis with histidine decarboxylase as a marker. Brain Res. 1984;295(1):13–25.
Bhuiyan ME, Waki H, Gouraud SS, et al. Histamine receptor H1 in the nucleus tractus solitarii regulates arterial pressure and heart rate in rats. Am J Physiol Heart Circ Physiol. 2011;301(2):H523–9.
Hayes MR, Skibicka KP, Bence KK, et al. Dorsal hindbrain 5’-adenosine monophosphate-activated protein kinase as an intracellular mediator of energy balance. Endocrinology. 2009;150(5):2175–82.
Pai N, Deng C, Vella S-L, et al. Are there different neural mechanisms responsible for three stages of weight gain development in anti-psychotic therapy: temporally based hypothesis. Asian J Psychiatr. 2012;5(4):315–8.
Huang XF, Han M, Huang X, et al. Olanzapine differentially affects 5-HT2Aand2C receptor mRNA expression in the rat brain. Behav Brain Res. 2006;171(2):355–62.
Albaugh VL, Henry CR, Bello NT, et al. Hormonal and metabolic effects of olanzapine and clozapine related to body weight in rodents. Obesity. 2006;14(1):36–51.
van der Zwaal EM, Merkestein M, Lam YK, et al. The acute effects of olanzapine on ghrelin secretion, CCK sensitivity, meal size, locomotor activity and body temperature. Int J Obesity. 2012;36(2):254–61.
Stefanidis A, Verty ANA, Allen AM, et al. The role of thermogenesis in antipsychotic drug-induced weight gain. Obesity. 2008;17(1):16–24.
Cuerda C, Merchan-Naranjo J, Velasco C, et al. Influence of resting energy expenditure on weight gain in adolescents taking second-generation antipsychotics. Clin Nutr. 2011;30(5):616–23.
Sharpe JK, Byrne NM, Stedman TJ, et al. Resting energy expenditure is lower than predicted in people taking atypical antipsychotic medication. J Am Diet Assoc. 2005;105(4):612–5.
Skouroliakou M, Giannopoulou I, Kostara C, et al. Comparison of predictive equations for resting metabolic rate in obese psychiatric patients taking olanzapine. Nutrition. 2009;25(2):188–93.
Blessing WW, Zilm A, Ootsuka Y. Clozapine reverses increased brown adipose tissue thermogenesis induced by 3,4-methylenedioxymethamphetamine and by cold exposure in conscious rats. Neuroscience. 2006;141(4):2067–73.
Monda M, Viggiano A, Viggiano E, et al. Quetiapine lowers sympathetic and hyperthermic reactions due to cerebral injection of orexin A. Neuropeptides. 2006;40(5):357–63.
He M, Zhang Q, Wang HQ, et al. Olanzapine treatment and time-dependent changes of hypothalamic AMPK-ACC-CPT1 signalling, food intake and body weight in rats [abstract no.POS-WED-077]. 32nd Australian Neuroscience Society Annual meeting; 2012 Jan 29–Feb 1; Gold Coast.
Seale P, Lazar MA. Brown fat in humans: turning up the heat on obesity. Diabetes. 2009;58(7):1482–4.
Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404(6778):652–60.
Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293(2):E444–52.
Whittle AJ, Carobbio S, Martins L, et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149(4):871–85.
Lopez M, Varela L, Vazquez MJ, et al. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med. 2010;16(9):1001–8.
Virtue S, Feldmann H, Christian M, et al. A new role for lipocalin prostaglandin d synthase in the regulation of brown adipose tissue substrate utilization. Diabetes. 2012;61(12):3139–47.
Miller DD. Atypical antipsychotics: sleep, sedation, and efficacy. Prim Care Companion J Clin Psychiatry. 2004;6(Suppl 2):3–7.
Reynolds GP, Kirk SL. Metabolic side effects of antipsychotic drug treatment—pharmacological mechanisms. Pharmacol Ther. 2010;125(1):169–79.
Muench J, Hamer AM. Adverse effects of antipsychotic medications. Am Fam Physician. 2010;81(5):617–22.
Nowakowska E, Chodera A, Kus K. Influence of olanzapine on cognitive functions and catalepsy in rats after single and chronic administration. Pol J Pharmacol. 1999;51(4):295–300.
Lenhard JM. Lipogenic enzymes as therapeutic targets for obesity and diabetes. Curr Pharm Des. 2011;17(4):325–31.
Skrede S, Fernø J, Vázquez MJ, et al. Olanzapine, but not aripiprazole, weight-independently elevates serum triglycerides and activates lipogenic gene expression in female rats. Int J Neuropsychopharmacol. 2012;15(02):163–79.
Vestri HS, Maianu L, Moellering DR, et al. Atypical antipsychotic drugs directly impair insulin action in adipocytes: Effects on glucose transport, lipogenesis, and antilipolysis. Neuropsychopharmacology. 2007;32(4):765–72.
Yang L-H, Chen T-M, Yu S-T, et al. Olanzapine induces SREBP-1-related adipogenesis in 3T3-L1 cells. Pharmacol Res. 2007;56(3):202–8.
Albaugh VL, Judson JG, She P, et al. Olanzapine promotes fat accumulation in male rats by decreasing physical activity, repartitioning energy and increasing adipose tissue lipogenesis while impairing lipolysis. Mol Psychiatry. 2011;16(5):569–81.
Scherer T, Buettner C. Yin and Yang of hypothalamic insulin and leptin signaling in regulating white adipose tissue metabolism. Rev Endocr Metab Disord. 2011;12(3):235–43.
Pardridge WM. Blood–brain barrier delivery. Drug Discov Today. 2007;12(1–2):54–61.
Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89(6):2595–600.
Després J-P. Abdominal obesity: the most prevalent cause of the metabolic syndrome and related cardiometabolic risk. Eur Heart J Suppl. 2006;8(suppl B):B4–12.
De Hert M, Cohen D, Bobes J, et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry. 2011;10(2):138–51.
De Hert M, Detraux J, van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2012;8(2):114–26.
De Hert M, Vancampfort D, Correll CU, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry. 2011;199(2):99–105.
Gothefors D, Adolfsson R, Attvall S, et al. Swedish clinical guidelines—prevention and management of metabolic risk in patients with severe psychiatric disorders. Nord J Psychiatry. 2010;64(5):294–302.
De Hert M, Dekker JM, Wood D, et al. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). Eur Psychiatry. 2009;24(6):412–24.
Hasnain M, Vieweg WV, Fredrickson SK, et al. Clinical monitoring and management of the metabolic syndrome in patients receiving atypical antipsychotic medications. Prim Care Diabetes. 2009;3(1):5–15.
Cooper GD, Harrold JA, Halford JCG, et al. Chronic clozapine treatment in female rats does not induce weight gain or metabolic abnormalities but enhances adiposity: Implications for animal models of antipsychotic-induced weight gain. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(2):428–36.
Acknowledgments
The study is supported by an Australian National Health and Medical Research Council (NHMRC) grant to Xu-Feng Huang and Chao Deng (ID 635231). Meng He is supported by the China Scholarship Council—University of Wollongong Joint Postgraduate Scholarship. Prof. Huang, Ms. He and A/Prof. Deng have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, M., Deng, C. & Huang, XF. The Role of Hypothalamic H1 Receptor Antagonism in Antipsychotic-Induced Weight Gain. CNS Drugs 27, 423–434 (2013). https://doi.org/10.1007/s40263-013-0062-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-013-0062-1