Abstract
Heart failure is a common disease state that can be encountered at different stages in the course of a COVID-19 patient presentation. New or existing heart failure in the setting of COVID-19 can present a set of unique challenges that can complicate presentation, management, and prognosis. A careful understanding of the hemodynamic and diagnostic implications is essential for appropriate triage and management of these patients. Abnormal cardiac biomarkers are common in COVID-19 and can stem from a variety of mechanisms that involve the viral entry itself through the ACE2 receptors, direct cardiac injury, increased thrombotic activity, stress cardiomyopathy, and among others. The cytokine storm observed in this pandemic can be a culprit in many of the observed mechanisms and presentations. A correct understanding of the two-way interaction between heart failure medications and the infection as well as the proposed COVID-19 medications and heart failure can result in optimal management. Guideline-directed medical therapy for heart failure should not be interrupted for theoretical concerns but rather based on tolerance and clinical presentation. Initiating specific cardiac or heart failure medications to prevent the infection or mitigate the disease is also not an evidence-based practice at this time. Heart failure patients on advanced therapies including those with heart transplantation will particularly benefit from involving the advanced heart failure team members in the overall management if they contract the virus.
Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. It was identified in December 2019 in Wuhan, China, and has evolved into a global health crisis. The full spectrum of SARS-CoV-2 infections in humans is not fully understood yet, but is being heavily studied. Patients with previously established comorbidities such as heart failure (HF) are at a particularly high risk of morbidity and mortality from this viral infection. In this review, we explore the risks associated with COVID-19 infection in HF patients and address unique aspects related to HF management in the acute and chronic settings when complicating the infection itself.
Heart failure patients and the risk of COVID-19 infection
In addition to older age, chronic comorbidities increase the risk of severe COVID-19 infection as well as its fatality. The overall global mortality rate of around 6.9% in COVID-19 is already significantly higher than that reported for the seasonal flu [2, 3] This becomes particularly concerning for patients with existing cardiovascular disease who generally experience worse prognosis and a mortality rate of over 10% in some reports [4]. Based on earlier analyses, cardiovascular disease (CVD) was statistically more prevalent in patients who die from the infection; a recent study from China that included 416 patients showed that 19% had signs of previous cardiac damage (defined as blood levels of cardiac biomarkers, namely high-sensitivity troponin I (hs-TNI) above the 99th-percentile upper reference limit of normal) and these patients were significantly more likely to experience mortality (51.2% vs 4.5%; P < 0.001) [5]. A more recent large global observational study that included 169 hospitals from three continents and close to 9000 patients found coronary artery disease and congestive heart failure (mortality of 15.3%, vs. 5.6% among those without heart failure; CI, 1.62 to 3.79) as independent predictors of in-hospital death [6].
HF patients are at especially increased risk due to their reduced immunity, general frailty, and reduced hemodynamic ability to cope with more severe infections. It was reported that in HF patients, monocytes seem to produce more TNF-α and less IL-10 than healthy subjects [7], which in combination with the widespread systemic inflammatory response associated with severe COVID-19 infections requires enhanced cardiac performance and high cardiac output, something that HF patients are generally incapable of.
Clinical and hemodynamic implications of COVID-19 infection in HF patients
Exacerbation of chronic HF
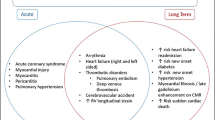
Lessons from the previous coronavirus and influenza epidemics suggest that viral infections can exacerbate a preexisting HF, with multiple studies showing an increase in HF re-hospitalization during influenza-like illness seasons [8]. With the more aggressive COVID-19 infection, HF patients are at a considerable higher risk of acute exacerbations, and multiple mechanisms may be responsible for triggering and aggravating this process (Fig. 1).
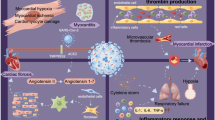
Acute infections result in the release of proinflammatory cytokines and the recruitment of proinflammatory macrophages and granulocytes, leading to a severe inflammatory storm which may exaggerate the initial injury [9]. In combination with the increased metabolic demand, this can lead to cardiac depression and either new-onset HF or acute decompensation of chronic HF [10]. Another contributor to cardiac depression could be the coagulation dysfunction induced by the sepsis [11]. Intensive care studies with COVID-19 patients from multiple European centers have shown high rates of coagulation abnormalities and thrombotic events [ 12, 13]. In another retrospective analysis from New York hospitals that included over 2000 critically ill patients, systemic anticoagulation was associated with prolonged survival particularly in mechanically ventilated patients [14]. Specific patient tailored algorithms have thus been suggested utilizing intensified pharmacologic prophylactic or full dose anticoagulation based on clinical presentation and biomarkers [15]. Close monitoring of anticoagulation in the LVAD population has also been recommended given the aforementioned high risk of thrombosis in this pandemic [16].
It was also reported that in 15–29% of COVID-19 patients, the virus causes kidney impairment in the setting of acute kidney injury [17, 18], which may lead to volume overload that may exacerbate a preexciting chronic HF.
Heart failure as a manifestation of COVID-19 infection in previously healthy individuals
New onset of HF was observed in as much as a quarter of hospitalized COVID-19 patients; and in as much as one-third of those admitted to the intensive care unit (ICU) [17, 19], despite not having a history of HF. This could be due to the direct effect of the virus or the systemic inflammation on the heart. Severe acute myocarditis can be a manifestation of the infection resulting in cardiogenic shock, which can then result in multi-organ dysfunction syndrome (MODS) and death [20]. Moreover, the prothrombotic state previously discussed can result in pulmonary embolism and thus acute right ventricular failure. The use of temporary heart pumps such as the Impella may be useful in these scenarios.
Stress cardiomyopathy-like picture can also be seen due to the generalized inflammatory response and sympathetic activation, resulting in a more classic acute HF decompensation with elevated filling pressures and pulmonary edema [21].
Hemodynamic and diagnostic implications in patients with COVID-19
Elevated natriuretic peptides suggest HF with a worse prognosis of COVID-19 and warrant at least an echocardiogram to further assess cardiac function [22]. If HF is present as part of the infectious process, optimizing loading conditions is critical. Minimal or very cautious use of IV fluids as well as inotropes rather than vasopressors is examples of how management can differ in these cases. When uncertain, hemodynamics are best assessed through pulmonary artery catheterization, especially if the diagnosis will change the management.
Markers of cardiac injury (CK-MB and Troponin) seem to rise significantly more in patients with CVD risk factors and are associated with significantly higher mortality. Multiple studies from Wuhan in China reported acute cardiac injury in 17–23% of the population, with overall worse prognosis and outcomes [17, 18].
Table 1 summarizes several studies from 3 continents (Asia, North America, Europe) that looked at the prevalence of preexisting cardiovascular comorbidities, septic shock, ICU admission, cardiac injury, and case fertility rate [17,18,19, 23,24,25,26,27,28,29,30,31,32].
Generally, the combination between HF and septic shock would lead to higher mortality rate of 70 to 90% when compared with 20% in septic patients without cardiovascular impairment [33]. Available reports estimated that vasoactive agents were required in 35% of the patients in a study from China and up to 67% of the critically ill patients in a study from the USA, although most of them did not present with evidence of shock [18, 19].
Furthermore, a careful clinical assessment of signs of hypervolemia or hypoperfusion is important in all COVID- 19 patients. In the case of cardiogenic shock requiring advanced therapy, it potentially means veno-arterial as opposed to veno-venous cannulation. Recently, cardiogenic shock was reported by Fried et al. as a complication during the treatment of COVID 19-related respiratory failure by veno-venous extra-corporeal membranous oxygenation (VV-ECMO), in which more advanced circulatory support with a low flow veno-arterial cannulation was required, highlighting the possible efficacy of such relatively low level of circulatory support without inducing LV distension in patients with COVID-19-related cardiopulmonary shock [34]. On the other hand, the severe respiratory illness induced by COVID-19 (Acute respiratory distress syndrome (ARDS), parenchymal lung disease) may lead to pulmonary hypertension and subsequent right-sided HF [35]. Most importantly, multi-disciplinary approach to these patients with collaboration between HF cardiologists, cardiac surgeons, and intensivists is invaluable. Finally, it is important to be aware of the risk of transmission and exposure at times of a pandemic and thus only perform imaging and laboratory testing when indicated.
Figure 2 summarizes an approach to COVID-19 patients with evidence of myocardial injury [36].
Heart failure medications in the setting of COVID-19 infection
COVID-19 mechanism of cellular entry has implicated an important class of medications that are part of the Guideline Directed Medical Therapy (GDMT) for heart failure with reduced ejection fraction (HFrEF). Similar to the previously known respiratory coronaviruses, SARS-CoV-2 spike glycoproteins bind to angiotensin-converting enzyme 2 (ACE2) receptors on the cell’s outer surface [37]. These receptors are largely in the lungs and small intestines, but have also been reported to be present in the heart [38]. With the emergence of the current pandemic, this known pharmacology led to several editorials and media reports questioning the associated risks in patients taking ACEI/ARB or suggesting that patients should prophylactically stop taking these medications [39]. Such concerns stem from the theory that patients on these medications are likely to have upregulated ACE2 receptors and thus are more prone to viral uptake or worse outcomes if they contract the infection.
The interplay of the virus with the ACE2 receptor and ACEI/ARB medications is rather more complex than a pharmacologically induced overexpression of a receptor and a resultant more viral susceptibility. First, while ACE and ACE2 are similar their active sites are different and they do not directly affect each other’s activity. Data from animal and human models are conflicting regarding the effect of ACE or even ARBs on the expression and activity of ACE2 [40,41,42]. Second, we still do not have data on the effect of these drugs on lung-specific expression of ACE2. In addition, even if we assume that RAAS inhibitors upregulate ACE2 levels, we do not have data to conclude that this will predispose to more entry of the virus in humans. Through converting angiotensin II to angiotensin 1–7, ACE2 in fact partially reverses the effects of the RAAS and its known detrimental outcomes in not only HF (vasoconstriction, myocardial remodeling) but also its potential role in inducing acute lung injury; therefore, attenuating the effects of an overactivated RAAS. This downregulation of RAAS has been used to explain the benefits of ACE2 in protection from severe lung injury in animal models [43, 44]. In a recent case series that included 363 hypertensive patients hospitalized with COVID-19, exposure to ACEI/ARB did not affect worsening disease or mortality [45]. In fact, there are currently ongoing randomized controlled trials to explore a potential benefit for losartan in hospitalized and non-hospitalized COVID-19 patients [46, 47]. A number of physician groups and professional societies recently considered the available data and the well-documented effect of discontinuing ACEI/ARB therapy and advised against changing clinical practice for the purpose of mitigating the pandemic and recommended to continue treatment based on standard indications [48].
COVID-19 suggested medications and their possible interaction with HF population
Based on in vitro data and a controversial recent trial, hydroxychloroquine has gained widespread adoption as part of institutional protocols to manage the COVID-19 infection [49, 50]. Both chloroquine and hydroxychloroquine block a potassium channel that can prolong the QTc and has been implicated in cases of torsades de pointes (TdP) and sudden cardiac death. This will become particularly relevant if combined with the other currently suggested treatments including the QTc prolonging azithromycin and lopinavir/ritonavir (all three medications are on CredibleMeds QTc prolonging list) [51]. While favipiravir has only been associated with QTc prolongation at high doses used to treat Ebola virus and not the current doses used for treatment in this pandemic, careful monitoring is still warranted given the relatively inadequate clinical experience with this agent [52, 53]. HF patients can be at particular risk for sudden cardiac death in the setting of the structural heart disease itself. This is in addition to being on concomitant proarrhythmic agents in advanced stages, as well as the high prevalence of antiarrhythmics and other concomitant medications that can pause challenging drug-drug interactions and in some cases, increase the chances of QTc prolongation. In fact, many hospitalized HF patients are likely to be categorized as medium to high risk based on the Tisdale score, which is a validated risk score to predict drug-associated QTc prolongation [54], this is due to several factors that include the disease state itself, being on a loop diuretic, frequent electrolyte abnormalities, and the aforementioned high prevalence of concomitant QTc prolonging medications. Moreover, heart failure patients on advanced therapies may also be on medications that interact with suggested COVID-19 regimens. For example, patients with left ventricular assist devices (LVAD) are generally on anticoagulation therapy with a vitamin k antagonist (VKA) such as warfarin. This can pose a drug-drug interaction with the some of the COVID-19 antivirals. In addition, patients who are post-heart transplantation will also be on an immunosuppressive regimen that is metabolized through some of cytochrome p450 enzymes that are inhibited by the antivirals. Table 2 summarizes selected drug-drug interactions between the suggested COVID-19 medications and potential cardiovascular and transplant medications that are commonly used in HF and transplant patients [55, 56].
Caring for HF patients while minimizing the risk of COVID-19 infection
Care pathways and delivery
Remote monitoring
The Heart Failure Society of America released a statement addressing how the care for HF patients has changed in the era of the COVID-19 pandemic [57]. Outpatient clinic visits are reduced in order to minimize patients’ exposure and transmission of the disease. This is paralleled by establishing virtual visits through video calls to ensure that the wide spectrum of HF patients and even mechanical circulatory support and heart transplant are still followed up properly and safely. This type of patients’ follow-up represents a win-win situation for both physicians and patients in the setting of a pandemic, and seems to be associated with better compliance with follow-up appointments. Gorodeski et al. evaluated no-show rates of HF patients in the HF clinic 7 days post-hospitalization and compared personal with virtual visits rates of 51% versus 34.6%, respectively. No significant differences in hospital readmission, emergency room visits, or death were observed between the two groups [57]. Although full physical examination cannot be conducted through virtual visits, it is feasible to inspect for clinical signs of volume overload such as lower extremity edema, or even jugular venous distention, which have been previously reported to correlate with invasive monitoring [58]. When these are accompanied with home monitoring and charting of daily weights and vitals including heart rate, blood pressure and oxygen saturation can all aid in assessing symptoms as well as guide any potential medication adjustments and up-titration. More assessments could be done, including exercise intolerance and NYHA functional classification.
Another technology that can potentially be of particular benefit at unprecedented times such as during this pandemic is remote monitoring of pulmonary artery pressure through technologies such as CardioMEMS (St Jude Medical, Inc.) or other similar implantable sensors. Pressure guide, physician-directed, patient self-management has been previously shown to not only reduce heart failure related hospitalizations but also lead to higher frequency of medication adjustments including neurohormonal antagonists [59]. The availability of this technology, as well as adapting to it, can be challenging in certain age groups or populations.
A recent Danish nationwide database that compared the incidence of new-onset HF and hospitalizations for HF before and after the lockdown for COVID-19 reported a significantly lower incidence during the lockdown period [60]. This raise concerns that HF patients who absolutely need medical attention may be avoiding it in fear of contracting the virus. Moreover, continued access to in-person visits and use of intravenous diuretics when necessary are essential to prevent decompensation despite existence of telemedicine or other strategies [61]. Therefore, it is important to raise awareness on the importance of seeking medical attention including hospital care or referral to heart failure specialists when necessary even during the pandemic.
Heart failure and self-care behaviors
In the era of a pandemic, and with emphasis on social distancing, the importance of self-care for HF patients is more than ever. Hospitals confer a higher risk of infections, and hospitalizations for HF carry a worse long-term prognosis. Adherence to medications and low-salt diet can be the difference maker in this regard. Careful attention to symptoms as well as daily weights can alert patients, family members, and healthcare providers to early worsening of chronic HF, leading to early adjustments that can keep them safely out of the hospital.
In addition to these self-care behaviors, community and institutional pharmacies can play a role by providing mail order or home delivery services of medications. This can result in less unnecessary exposure of the patient to the community. Another example is anticoagulation monitoring for patients who are on vitamin k antagonists and require blood checks. Creative approaches to such patients including lab draws followed by telemedicine or even drive through international normalized ratio (INR) checks are all strategies that can avoid physical clinic visits and potentially reduce risk of exposure particularly for high-risk patients. Drug shortages have also been a concern in this pandemic, and pharmacists can play a role by assuring patients, designing strategies to ensure appropriate use, and providing alternatives through discussions with physicians when necessary [62].
Unique populations
Heart transplant patients
Generally, infections are always a concern in post-heart transplantation given the need to use immunosuppression to prevent rejection and preserve graft function. While immunosuppression increases the likelihood of developing viral infections such as cytomegalovirus and herpes, acute respiratory viral infections are less pronounced [63]. To date, it is unclear if heart transplant patients are at increased risk of acquiring the novel virus. In a small study from China [64], Ren et al. observed 87 heart transplant recipients during the pandemic and found that only four of them had upper respiratory infections, which were unrelated to COVID-19 in most of these patients. It is very likely that the perceived risk of infection in these patients led them to proactively use measures that can limit the transmission of the virus, such as wearing a mask for example.
When infected by the virus, immunosuppression may influence the typical clinical presentation of COVID-19 patients who are also heart transplant recipients, resulting in unusual symptoms such as gastrointestinal manifestations [65]. However, it was reported in many cases around the world that clinical manifestations were similar to what has been encountered in non-immunosuppressed patients with COVID-19 [34, 66]. The clinical picture in reported cases from China was accompanied by mild to severe symptoms at presentation, typical imaging findings, and identical laboratory findings with the exception of higher C-reactive protein (CRP) and lower lymphocyte counts compared with non-heart transplant patients. Reported cases from a European transplantation center showed that solid organ recipients with COVID-19 had a more severe clinical course and high complications rates when compared with the general populations [65]. Additionally, COVID-19 infection with myocarditis in a heart transplant recipient can mimic acute allograft rejection. Noninvasive tests like gene profiling or donor-derived cell-free DNA can help differentiate the two entities [67]. A recent case series of 28 heart transplant recipients and a confirmed diagnosis of COVID-19 from New York reported an alarmingly high casa fatality rate of 25%. However, it is important to note here that the median age of these patients was 64 years (interquartile range (IQR), 53.5–70.5 years) and more than half of them already had cardiac allograft vasculopathy (CAV). The approach to treatment followed in these patients included reducing immunosuppression in most of these patients [68]. This also brings up the question as to the best strategy of treatment for this unique population.
The International Society for Heart and Lung Transplantation (ISHLT) guidelines suggest holding the immunosuppressive drugs like mycophenolate mofetil or azathioprine in moderate to severe presentations of COVID-19 [69]. For COVID-19 survivors on the waiting list, it is crucial to obtain two negative tests after a 14-day interval post-diagnosis in order to proceed with transplantation under special considerations according to the acuity of the disease and organ availability.
For donors, current ISHLT recommendations support testing for the novel virus if the test is available. Donors should be excluded if the test is positive or if they have imaging findings suggestive of viral pneumonitis, which can sometimes precede symptoms and even the positive PCR test. Moreover, current concerns about nosocomial transmissions warrant a second test for previously negative tested donors when organ recovery starts [70].
Ventricular assist devices
Although cellular immunity was reported to be compromised among long-term LVAD recipients, [71, 72] there is no clear evidence suggesting that they are at increased risk of acquiring the virus. It is also known that the inflammatory profile is significantly altered in HF patients supported by LVAD therapy, who generally have elevated cytokines [73]. This should be taken into consideration when clinically evaluating LVAD patients presenting with COVID-19 or utilizing their inflammatory and cardiac biomarkers for treatment or prognosis.
Although cardiac output provided through the assist device theoretically remains steady even in the setting of a systemic infection, optimizing preload and afterload is very important to sustain it. If hemodynamics are compromised, various LVAD-related complications can ensue, including right ventricular failure and pump thrombosis, as well as low flow and suction events [74].
Early case reports of COVID-19 infections in LVAD patients highlighted refractory hypoxemia leading to tracheostomy, and right ventricular failure leading to multi-organ dysfunction [75, 76]. An important management issue in these cases was the limited implementation of prone ventilation, a technique known to be beneficial in managing ARDS with a refractory hypoxemia, due to fears that the outflow graft and/or the driveline may be compressed in such position, or that it would impair venous return, thus worsening RV hemodynamics resulting in progressive HF.
Conclusion
Heart failure is common and may be encountered de novo as part of the clinical course of COVID-19 or in those with preexisting cardiac disease. It is thus imperative to understand the diverse interactions between this disease state and the virus to optimize the management of these patients. A multi-disciplinary approach that involves members of the heart failure team may result in optimal understanding and management of chronic heart failure patients including those on advanced therapies in the context of this pandemic.
References
Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR (2020 Feb) Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents 17:105924
COVID-19 Map [Internet] (2020) Johns Hopkins Coronavirus Resource Center. [cited 27 April 2020]. Available from: https://coronavirus.jhu.edu/map.html. Accessed 27 Apr 2020
WHO Director-General’s opening remarks at the media briefing on COVID-19 - 3 March 2020 [Internet]. Who.int. (2020). Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19%2D%2D-3-march-2020. Accessed 27 Apr 2020
Novel CP (2020) The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 41(2):145
Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H (2020) Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 5(7):802–810. https://doi.org/10.1001/jamacardio.2020.0950
Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN (2020) Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med 382(25):e102. https://doi.org/10.1056/NEJMoa2007621
Ng TM, Toews ML (2016) Impaired norepinephrine regulation of monocyte inflammatory cytokine balance in heart failure. World J Cardiol 8(10):584–589
Kytömaa S, Hegde S, Claggett B, Udell JA, Rosamond W, Temte J, Nichol K, Wright JD, Solomon SD, Vardeny O (2019) Association of influenza-like illness activity with hospitalizations for heart failure: the atherosclerosis risk in communities study. JAMA Cardiol 4(4):363–369
Tufan A, GÜLER AA, Matucci-Cerinic M (2020) COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk J Med Sci 50(SI-1):620–632
Kumar A, Parrillo JE, Kumar A (2002) Clinical review: myocardial depression in sepsis and septic shock. Crit Care 6(6):500
Dellinger RP (2003) Inflammation and coagulation: implications for the septic patient. Clin Infect Dis 36(10):1259–1265
Klok FA, Kruip MJ, Van der Meer NJ, Arbous MS, Gommers DA, Kant KM, Kaptein FH, van Paassen J, Stals MA, Huisman MV, Endeman H (2020) Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res 191:148–150. https://doi.org/10.1016/j.thromres.2020.04.041
Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, Fafi-Kremer S (2020) High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 46(6):1089–1098. https://doi.org/10.1007/s00134-020-06062-x
Paranjpe I, Fuster V, Lala A, Russak A, Glicksberg BS, Levin MA, Charney AW, Narula J, Fayad ZA, Bagiella E, Zhao S (2020) Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol 76(1):122–124. https://doi.org/10.1016/j.jacc.2020.05.001
Atallah B, Mallah SI, AlMahmeed W (2020) Anticoagulation in COVID-19. Eur Heart J Cardiovasc Pharmacother 6(4):260–261. https://doi.org/10.1093/ehjcvp/pvaa036
DeFilippis EM, Reza N, Donald E, Givertz MM, Lindenfeld J, Jessup M (2020) Considerations for heart failure care during the coronavirus disease 2019 (COVID-19) pandemic. JACC Heart Fail S2213-1779(20)30273–0. https://doi.org/10.1016/j.jchf.2020.05.006
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395(10229):1054–1062. https://doi.org/10.1016/S0140-6736(20)30566-3
Yang X, Yu Y, Xu J, Shu H, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 8(5):475–481. https://doi.org/10.1016/S2213-2600(20)30079-5
Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M (2020) Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 323(16):1612–1614
Ruan Q, Yang K, Wang W, Jiang L, Song J (2020) Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 46(5):846–848. https://doi.org/10.1007/s00134-020-05991-x
Scally C, Abbas H, Ahearn T, Srinivasan J, Mezincescu A, Rudd A, Spath N, Yucel-Finn A, Yuecel R, Oldroyd K, Dospinescu C (2019) Myocardial and systemic inflammation in acute stress-induced (Takotsubo) cardiomyopathy. Circulation 139(13):1581–1592
Gao L, Jiang D, Wen XS, Cheng XC, Sun M, He B, You LN, Lei P, Tan XW, Qin S, Cai GQ (2020) Prognostic value of NT-proBNP in patients with severe COVID-19. Respir Res 21(1):1–7
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 323(11):1061–1069
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DS, Du B (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382(18):1708–1720
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Yu T (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395(10223):507–513
Fang Z, Yi F, Wu K, Lai K, Sun X, Zhong N, Liu Z (2020) Clinical characteristics of 2019 coronavirus pneumonia (COVID-19): an updated systematic review. medRxiv
Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G (2020) Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 323(16):1574–1581
Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, Holden KA, Read JM, Dondelinger F, Carson G, Merson L (2020) Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 369:m1985. https://doi.org/10.1136/bmj.m1985
Buckner FS, McCulloch DJ, Atluri V, Blain M, McGuffin SA, Nalla AK, Huang ML, Greninger AL, Jerome KR, Cohen SA, Neme S (2020) Clinical features and outcomes of 105 hospitalized patients with COVID-19 in Seattle, Washington. Clin Infect Dis ciaa632. https://doi.org/10.1093/cid/ciaa632
Almazeedi S, Al Youha S, Jamal MH, Al-Haddad M, Al-Muhaini A, Al-Ghimlas F, Al-Sabah S (2020) Clinical characteristics, risk factors and outcomes among the first consecutive 1,096 patients diagnosed with COVID-19: the Kuwait experience. medRxiv
Javanian M, Bayani M, Shokri M, Sadeghi-Haddad-Zavareh M, Babazadeh A, Yeganeh B, Mohseni S, Mehraein R, Sepidarkish M, Bijani A, Rostami A (2020) Clinical and laboratory findings from patients with COVID-19 pneumonia in Babol North of Iran: a retrospective cohort study. Rom J Intern Med;1(ahead-of-print) https://doi.org/10.2478/rjim-2020-0013
Merx MW, Weber C (2007) Sepsis and the heart. Circulation. 116(7):793–802
Fried JA, Ramasubbu K, Bhatt R, Topkara VK, Clerkin KJ, Horn E, Rabbani L, Brodie D, Jain SS, Kirtane A, Masoumi A (2020) The variety of cardiovascular presentations of COVID-19. Circulation 141(23):1930–1936. https://doi.org/10.1161/CIRCULATIONAHA.120.047164
Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, Brown TS, Der Nigoghossian C, Zidar DA, Haythe J, Brodie D (2020) Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol 75(18):2352–2371
Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD (2018) Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 72(18):2231–2264
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271–280.e8. https://doi.org/10.1016/j.cell.2020.02.052
Tikellis C, Thomas MC (2012) Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int J Pept 2012:1–8
Fang L, Karakiulakis G, Roth M (2020) Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 8(4):e21. https://doi.org/10.1016/S2213-2600(20)30116-8
Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM (2004) Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J 383(1):45–51
Hamming I, Van Goor H, Turner AJ, Rushworth CA, Michaud AA, Corvol P, Navis G (2008) Differential regulation of renal angiotensin-converting enzyme (ACE) and ACE2 during ACE inhibition and dietary sodium restriction in healthy rats. Exp Physiol 93(5):631–638
Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE (2005) Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 111(20):2605–2610
Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA (2005) Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436(7047):112–116
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L (2005) A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med 11(8):875–879
Li J, Wang X, Chen J, Zhang H, Deng A (2020) Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol 5(7):1–6. https://doi.org/10.1001/jamacardio.2020.1624
ClinicalTrials.gov. Randomized controlled trial of losartan for patients with COVID-19 not requiring hospitalization. Identifier: NCT04311177. March 17, 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT04311177. Accessed 27 Apr 2020
ClinicalTrials.gov. Randomized controlled trial of losartan for patients with COVID-19 requiring hospitalization. Identifier: NCT04312009. March 17, 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT04312009. Accessed 27 Apr 2020
HFSA/ACC/AHA statement addresses concerns re: using RAAS antagonists in COVID-19 - American College of Cardiology [Internet]. American College of Cardiology. 2020. Available from: https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19. Accessed 27 Apr 2020
Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30(3):269–271
Gautret P, Lagier JC, Parola P, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Dupont HT, Honoré S (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 56(1):105949. https://doi.org/10.1016/j.ijantimicag.2020.105949
[Internet]. Crediblemeds.org. [cited 1 May 2020]. Available from: https://www.crediblemeds.org/pdftemp/pdf/CombinedList.pdf. Accessed 1 May 2020
Kumagai Y, Murakawa Y, Hasunuma T, Aso M, Yuji W, Sakurai T, Noto M, Oe T, Kaneko A (2015) Lack of effect of favipiravir, a novel antiviral agent, on QT interval in healthy Japanese adults. Int J Clin Pharmacol Ther 53(10):866–874
Chinello P, Petrosillo N, Pittalis S, Biava G, Ippolito G, Nicastri E, INMI Ebola Team. QTc interval prolongation during favipiravir therapy in an Ebolavirus-infected patient. PLoS Negl Trop Dis 11(12):e0006034. https://doi.org/10.1371/journal.pntd.0006034
Tisdale JE, Jaynes HA, Kingery JR, Mourad NA, Trujillo TN, Overholser BR, Kovacs RJ (2013) Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Cir Cardiovasc Qual Outcomes 6(4):479–487
Hayeshi R, Masimirembwa C, Mukanganyama S, Ungell AL (2006) The potential inhibitory effect of antiparasitic drugs and natural products on P-glycoprotein mediated efflux. Eur J Pharm Sci 29(1):70–81
Somer M, Kallio J, Pesonen U, Pyykkö K, Huupponen R, Scheinin M (2000) Influence of hydroxychloroquine on the bioavailability of oral metoprolol. Br J Clin Pharmacol 49(6):549–554
Gorodeski EZ, Goyal P, Cox ZL, Thibodeau JT, Reay RE, Rasmusson K, Rogers JG, Starling RC (2020) Virtual visits for care of patients with heart failure in the era of COVID-19: a statement from the Heart Failure Society of America. J Card Fail 26(6):448–456. https://doi.org/10.1016/j.cardfail.2020.04.008
Abnousi F, Kang G, Giacomini J, Yeung A, Zarafshar S, Vesom N, Ashley E, Harrington R, Yong C (2019) A novel noninvasive method for remote heart failure monitoring: the EuleriAn video Magnification apPLications In heart Failure studY (AMPLIFY). NPJ Digit Med 2(1):1–6
Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS (2011) Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial [published correction appears in Lancet. 2012 Feb 4;379(9814):412]. Lancet 377(9766):658–666. https://doi.org/10.1016/S0140-6736(11)60101-3
Andersson C, Gerds T, Fosbøl E, Phelps M, Andersen J, Lamberts M, Holt A, Butt JH, Madelaire C, Gislason G, Torp-Pedersen C (2020) Incidence of new-onset and worsening heart failure before and after the COVID-19 epidemic lockdown in Denmark: a nationwide cohort study. Circ Heart Fail 13(6):e007274. https://doi.org/10.1161/CIRCHEARTFAILURE.120.007274
UMAPATHI P, CUOMO K, RILEY S, HUBBARD A, MENZEL K, SAUER E, GILOTRA NA Transforming ambulatory heart failure care in the coronavirus disease-19 era: initial experience from a heart failure disease management clinic. J Card Fail 26(7):637–638. https://doi.org/10.1016/j.cardfail.2020.06.003
Badreldin HA, Atallah B (2020) Global drug shortages due to COVID-19: impact on patient care and mitigation strategies. Res Soc Adm Pharm S1551-7411(20)30569–6. https://doi.org/10.1016/j.sapharm.2020.05.017
Ambrosi P (2020) Comment on “Epidemiological and clinical characteristics of heart transplant recipients during the 2019 coronavirus outbreak in Wuhan, China” by Ren et al. J Heart Lung Transplant 39(7):729. https://doi.org/10.1016/j.healun.2020.04.002
Ren ZL, Hu R, Wang ZW, Zhang M, Ruan YL, Wu ZY, Wu HB, Hu XP, Hu ZP, Ren W, Li LC (2020) Epidemiological and clinical characteristics of heart transplant recipients during the 2019 coronavirus outbreak in Wuhan, China: a descriptive survey report. J Heart Lung Transplant 39(5):412–417. https://doi.org/10.1016/j.healun.2020.03.008
Fernández-Ruiz M, Andrés A, Loinaz C, Delgado JF, López-Medrano F, San Juan R, González E, Polanco N, Folgueira MD, Lalueza A, Lumbreras C (2020) COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant 20(7):1849–1858. https://doi.org/10.1111/ajt.15929
Li F, Cai J, Dong N (2020) First cases of COVID-19 in heart transplantation from China. J Heart Lung Transplant 39(5):496–497
DeFilippis EM, Farr MA, Givertz MM (2020) Challenges in heart transplantation in the era of COVID-19. Circulation 141(25):2048–2051. https://doi.org/10.1161/CIRCULATIONAHA.120.047096
Latif F, Farr MA, Clerkin KJ, Habal MV, Takeda K, Naka Y, Restaino S, Sayer G, Uriel N (2020) Characteristics and outcomes of recipients of heart transplant with coronavirus disease 2019. JAMA Cardiol e202159. https://doi.org/10.1001/jamacardio.2020.2159
Guidance for cardiothoracic transplant and ventricular assist device centers regarding the SARS CoV-2 pandemic [Internet]. Ishlt.org. (2020). Available from: https://ishlt.org/ishlt/media/documents/SARS-CoV-2_-Guidance-for-Cardiothoracic-Transplant-and-VAD-centers.pdf. Accessed 27 Apr 2020
Patel KJ, Kao T, Geft D, Czer L, Esmailian F, Kobashigawa JA, Patel JK (2020) Donor organ evaluation in the era of coronavirus disease 2019: a case of nosocomial infection. J Heart Lung Transplant 39(6):611–612. https://doi.org/10.1016/j.healun.2020.04.005
Ankersmit HJ, Tugudea S, Spanier T, Weinberg AD, Artrip JH, Burke EM, Flannery M, Mancini D, Rose EA, Edwards NM, Oz MC (1999) Activation-induced T-cell death and immune dysfunction after implantation of left-ventricular assist de vice. Lancet 354(9178):550–555
Kimball PM, Flattery M, McDougan F, Kasirajan V (2008 May 1) Cellular immunity impaired among patients on left ventricular assist device for 6 months. Ann Thorac Surg 85(5):1656–1661
Radley G, Pieper IL, Ali S, Bhatti F, Thornton CA (2018) The inflammatory response to ventricular assist devices. Front Immunol 9:2651
Kilic A, Acker MA, Atluri P (2015) Dealing with surgical left ventricular assist device complications. J Thorac Dis 7(12):2158–2164
Singh R, Domenico C, Rao S, Urgo K, Prenner S, Wald J, Atluri P, Birati EY (2020) Novel coronavirus disease 2019 in a patient on durable left ventricular assist device support. J Card Fail 26(5):438–439. https://doi.org/10.1016/j.cardfail.2020.04.007
Chau VQ, Oliveros E, Mahmood K, Singhvi A, Lala A, Moss N, Gidwani U, Mancini DM, Pinney SP, Parikh A (2020) The imperfect cytokine storm: severe COVID-19 with ARDS in patient on durable LVAD support. JACC Case Rep 2(9):1315–1320. https://doi.org/10.1016/j.jaccas.2020.04.001
Author information
Authors and Affiliations
Contributions
All named authors have seen and approved the final version of the manuscript. All authors contributed to the design and the writing of the manuscript.
Corresponding author
Ethics declarations
All material is original to this submission.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The manuscript, or part of it, has neither been published nor is currently under consideration for publication by any other journal.
Rights and permissions
About this article
Cite this article
Bader, F., Manla, Y., Atallah, B. et al. Heart failure and COVID-19. Heart Fail Rev 26, 1–10 (2021). https://doi.org/10.1007/s10741-020-10008-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-020-10008-2