Abstract
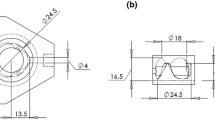
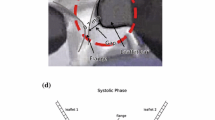
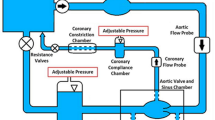
Aortic valve (AV) calcification is a highly prevalent disease with serious impact on mortality and morbidity. Although exact causes and mechanisms of AV calcification are unclear, previous studies suggest that mechanical forces play a role. Since calcium deposits occur almost exclusively on the aortic surfaces of AV leaflets, it has been hypothesized that adverse patterns of fluid shear stress on the aortic surface of AV leaflets promote calcification. The current study characterizes AV leaflet aortic surface fluid shear stresses using Laser Doppler velocimetry and an in vitro pulsatile flow loop. The valve model used was a native porcine valve mounted on a suturing ring and preserved using 0.15% glutaraldehyde solution. This valve model was inserted in a mounting chamber with sinus geometries, which is made of clear acrylic to provide optical access for measurements. To understand the effects of hemodynamics on fluid shear stress, shear stress was measured across a range of conditions: varying stroke volumes at the same heart rate and varying heart rates at the same stroke volume. Systolic shear stress magnitude was found to be much higher than diastolic shear stress magnitude due to the stronger flow in the sinuses during systole, reaching up to 20 dyn/cm2 at mid-systole. Upon increasing stroke volume, fluid shear stresses increased due to stronger sinus fluid motion. Upon increasing heart rate, fluid shear stresses decreased due to reduced systolic duration that restricted the formation of strong sinus flow. Significant changes in the shear stress waveform were observed at 90 beats/min, most likely due to altered leaflet dynamics at this higher heart rate. Overall, this study represents the most well-resolved shear stress measurements to date across a range of conditions on the aortic side of the AV. The data presented can be used for further investigation to understand AV biological response to shear stresses.
Similar content being viewed by others
References
Agmon Y, Khandheria BK, Meissner I, Sicks JR, O’Fallon WM, Wiebers DO, Whisnant JP, Seward JB, Tajik AJ (2001) Aortic valve sclerosis and aortic atherosclerosis: different manifestations of the same disease? Insights from a population-based study. J Am Coll Cardiol 38(3): 827–834
Balachandran K, Sucosky P, Jo H, Yoganathan AP (2009) Elevated cyclic stretch alters matrix remodeling in aortic valve cusps: implications for degenerative aortic valve disease. Am J Physiol Heart Circ Physiol 296(3): H756–H764
Bellhouse BJ, Reid KG (1969) Fluid mechanics of the aortic valve. Br Heart J 31(3): 391
Billiar KL, Sacks MS (2000) Biaxial mechanical properties of the natural and glutaraldehyde treated aortic valve cusp—Part I: experimental results. J Biomech Eng 122(1): 23–30
Butcher JT, Penrod AM, Garcia AJ, Nerem RM (2004) Unique morphology and focal adhesion development of valvular endothelial cells in static and fluid flow environments. Arterioscler Thromb Vasc Biol 24(8): 1429–1434
Butcher JT, Simmons CA, Warnock JN (2008) Mechanobiology of the aortic heart valve. J Heart Valve Dis 17(1): 62–73
Butcher JT, Tressel S, Johnson T, Turner D, Sorescu G, Jo H, Nerem RM (2006) Transcriptional profiles of valvular and vascular endothelial cells reveal phenotypic differences: influence of shear stress. Arterioscler Thromb Vasc Biol 26(1): 69–77
Carmody CJ, Burriesci G, Howard IC, Patterson EA (2006) An approach to the simulation of fluid-structure interaction in the aortic valve. J Biomech 39(1): 158–169
Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH (2007) Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol 49(25): 2379–2393
Cui W, Roberson DA, Chen Z, Madronero LF, Cuneo BF (2008) Systolic and diastolic time intervals measured from Doppler tissue imaging: normal values and Z-score tables, and effects of age, heart rate, and body surface area. J Am Soc Echocardiogr 21(4): 361–370
Davies PF, Spaan JA, Krams R (2005) Shear stress biology of the endothelium. Ann Biomed Eng 33(12): 1714–1718
De Hart J, Peters GW, Schreurs PJ, Baaijens FP (2003) A three-dimensional computational analysis of fluid-structure interaction in the aortic valve. J Biomech 36(1): 103–112
De Hart J, Peters GW, Schreurs PJ, Baaijens FP (2004) Collagen fibers reduce stresses and stabilize motion of aortic valve leaflets during systole. J Biomech 37(3): 303–311
Freeman RV, Otto CM (2005) Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 111(24): 3316–3326
Ge L, Sotiropoulos F (2010) Direction and magnitude of blood flow shear stresses on the leaflets of aortic valves: is there a link with valve calcification?. J Biomech Eng 132(1): 014505
Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA (1987) Heart rate and cardiovascular mortality: the Framingham study. Am Heart J 113(6):1489–1494
Ku DN, Giddens DP, Zarins CK, Glagov S (1985) Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis 5(3): 293–302
Leo HL, Dasi LP, Carberry J, Simon HA, Yoganathan AP (2006) Fluid dynamic assessment of three polymeric heart valves using particle image velocimetry. Ann Biomed Eng 34(6): 936–952
Levy RL, White PD et al (1946) Transient tachycardia; prognostic significance alone and in association with transient hypertension. Med Press Egypt 38(6): 207–212
Lindroos M, Kupari M, Heikkila J, Tilvis R (1993) Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 21(5): 1220–1225
Makhijani VB, Yang HQ, Dionne PJ, Thubrikar MJ (1997) Three-dimensional coupled fluid-structure simulation of pericardial bioprosthetic aortic valve function. ASAIO J 43(5): M387–M392
Markl M, Draney MT, Miller DC, Levin JM, Williamson EE, Pelc NJ, Liang DH, Herfkens RJ (2005) Time-resolved three-dimensional magnetic resonance velocity mapping of aortic flow in healthy volunteers and patients after valve-sparing aortic root replacement. J Thoracic Cardiovasc Surg 130(2): 456–463
Matsumoto Y, Adams V, Jacob S, Mangner N, Schuler G, Linke A (2010) Regular exercise training prevents aortic valve disease in low-density lipoprotein-receptor-deficient mice. Circulation 121(6): 759–767
Ming L, Zhen-huang K (1986) Study of the closing mechanism of natural heart valves. Appl Math Mech 7(10): 955–964
Morsi YS, Yang WW, Wong CS, Das S (2007) Transient fluid-structure coupling for simulation of a trileaflet heart valve using weak coupling. J Artif Organs 10(2): 96–103
Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD (1994) Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation 90(2): 844–853
Palatini P (1999) Elevated heart rate as a predictor of increased cardiovascular morbidity. J Hypertens Suppl 17(3): S3–S10
Palatini P, Casiglia E, Pauletto P, Staessen J, Kaciroti N, Julius S (1997) Relationship of tachycardia with high blood pressure and metabolic abnormalities: a study with mixture analysis in three populations. Hypertension 30(5): 1267–1273
Pate GE (2002) Association between aortic stenosis and hypertension. J Heart Valve Dis 11(5): 612–614
Peskin CS, Wolfe AW (1978) The aortic sinus vortex. Fed Proc 37(14): 2784–2792
Platt MO, Xing Y, Jo H, Yoganathan AP (2006) Cyclic pressure and shear stress regulate matrix metalloproteinases and cathepsin activity in porcine aortic valves. J Heart Valve Dis 15(5): 622–629
Rabkin SW (2005) The association of hypertension and aortic valve sclerosis. Blood Press 14(5): 264–272
Sacks MS, Schoen FJ, Mayer JE (2009) Bioengineering challenges for heart valve tissue engineering. Annu Rev Biomed Eng 11: 289–313
Saito M, Okayama H, Nishimura K, Ogimoto A, Ohtsuka T, Inoue K, Hiasa G, Sumimoto T, Higaki J (2008) Possible link between large artery stiffness and coronary flow velocity reserve. Heart 94(6): e20
Sarraf CE, Harris AB, McCulloch AD, Eastwood M (2002) Tissue engineering of biological cardiovascular system surrogates. Heart Lung Circ 11(3): 142–150 discussion 151
Smith KE, Metzler SA, Warnock JN (2010) Cyclic strain inhibits acute pro-inflammatory gene expression in aortic valve interstitial cells. Biomech Model Mechanobiol 9(1): 117–125
Stevens T, Rosenberg R, Aird W, Quertermous T, Johnson FL, Garcia JG, Hebbel RP, Tuder RM, Garfinkel S (2001) NHLBI workshop report: endothelial cell phenotypes in heart, lung, and blood diseases. Am J Physiol Cell Physiol 281(5): C1422–C1433
Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM (1997) Clinical factors associated with calcific aortic valve disease. Cardiovascular health study. J Am Coll Cardiol 29(3): 630–634
Sucosky P, Balachandran K, Elhammali A, Jo H, Yoganathan AP (2009) Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4- and TGF-beta1-dependent pathway. Arterioscler Thromb Vasc Biol 29(2): 254–260
Swanson M, Clark RE (1974) Dimensions and geometric relationships of the human aortic valve as a function of pressure. Circ Res 35(6): 871–882
Thubrikar M (1990) The aortic valve. Boca Raton, Florida
Topper JN, Gimbrone MA Jr (1999) Blood flow and vascular gene expression: fluid shear stress as a modulator of endothelial phenotype. Mol Med Today 5(1):40–46
Vesely I, Boughner D (1989) Analysis of the bending behaviour of porcine xenograft leaflets and of natural aortic valve material: bending stiffness, neutral axis and shear measurements. J Biomech 22(6–7): 655–671
Weinberg EJ, Kaazempur Mofrad MR (2008) A multiscale computational comparison of the bicuspid and tricuspid aortic valves in relation to calcific aortic stenosis. J Biomech 41(16): 3482–3487
Weinberg EJ, Mack PJ, Schoen FJ, Garcia-Cardena G, Kaazempur Mofrad MR (2010) Hemodynamic environments from opposing sides of human aortic valve leaflets evoke distinct endothelial phenotypes in vitro. Cardiovasc Eng 10(1): 5–11
Weston MW, LaBorde DV, Yoganathan AP (1999) Estimation of the shear stress on the surface of an aortic valve leaflet. Ann Biomed Eng 27(4): 572–579
Xing Y, Warnock JN, He Z, Hilbert SL, Yoganathan AP (2004) Cyclic pressure affects the biological properties of porcine aortic valve leaflets in a magnitude and frequency dependent manner. Ann Biomed Eng 32(11): 1461–1470
Yap CH, Dasi LP, Yoganathan AP (2010) Dynamic hemodynamic energy loss in normal and stenosed aortic valves. J Biomech Eng 132(2): 021005
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yap, C.H., Saikrishnan, N., Tamilselvan, G. et al. Experimental measurement of dynamic fluid shear stress on the aortic surface of the aortic valve leaflet. Biomech Model Mechanobiol 11, 171–182 (2012). https://doi.org/10.1007/s10237-011-0301-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-011-0301-7