Abstract
α1A-adrenergic receptor (α1A-AR) regulates the cardiac and peripheral vascular system through sympathetic activation, and α2A-AR and α2C-AR subtypes are essential for presynaptic feedback regulation of catecholamine release from the central and peripheral sympathetic nerve. Genetic variations in each human α-AR subtype gene have been identified and have been implicated in hypertension and cardiovascular disease. It is not yet clear whether these genetic variations actually have an effect on sympatho-vagal modulation. The aim of the present study was to evaluate the relation between the five representative genetic polymorphisms of α-AR subtypes (Arg347Cys of α1A-AR; C-1291G, Asn251Lys, and DraI RFLP of α2A-AR; and Del322–325 of α2C-AR) and autonomic nervous system (ANS) function in young and healthy Japanese males. One hundred forty-nine subjects were genotyped for each α-AR polymorphism, and underwent evaluation of ANS function by power spectral analysis of heart rate variability (HRV) during supine rest and in a standing position. In a supine position, the α1A-AR 347Cys allele was significantly associated with lower HRV sympathetic index (normalized low frequency power [LF(%)] and LF:HF ratio) and higher HRV parasympathetic index [HF(%)]. Meanwhile, subjects with the α2C-AR Del322–325 allele had markedly higher LF(%) and LF:HF ratio and lower HF(%) than noncarriers. Thus, the α1A-AR and α2C-AR genetic variations influence sympatho-vagal balance even in young and healthy normotensive states, which could be postulated to constitute an intermediate phenotype for future pathological episodes of various ANS dysfunction-related diseases.
Similar content being viewed by others
Introduction
α1- and α2-adrenergic receptors (α1A-, α1B-, α1D-, α2A-, α2B-, and α2C-AR) classically couple with a particular class of G proteins and mediate actions in the sympathetic nervous system. Among three subtypes of α1-ARs, the α1A-AR is the predominant subtype in both the heart and vasculature and is a major contributor involved in the sympathetic regulation of blood pressure and peripheral vascular resistance (Guimaraes and Moura 2001; Tanoue et al. 2002). Meanwhile, α2-ARs are important regulators of sympathetic tone, neurotransmitter release, blood pressure, and lipolysis in fat tissue (Lafontan and Berlan 1995; Philipp et al. 2002). They are widely expressed in the human central and peripheral nervous systems, and α2A- and α2C-AR are presynaptic receptor subtypes that contribute to feedback control of norepinephrine release from sympathetic nerves (Brede et al. 2002, 2003; Philipp et al. 2002).
The physiological and therapeutic responsiveness of α1- and α2-AR to agonists or antagonists exhibits interindividual variability (Dao et al. 1998; Eichler et al. 1989; Masuki et al. 2006), which suggests a relation to genetic variations in these receptors. The human α1A-, α2A-, and α2C-AR genes (ADRA1A, ADRA2A, ADRA2C) are located on chromosomes 8p21, 10q24–q26, and 4p16, respectively. Genetic polymorphisms have been identified for each α-AR subtype. One of the ADRA1A polymorphisms, the Arg347Cys (previously described as Arg492Cys) mutation, is located in the carboxyl terminus of the receptor (Shibata et al. 1996). This variant has no apparent effect on the functional properties in vitro (Shibata et al. 1996), and an initial report found no association between the Arg347Cys variant and hypertension (Xie et al. 1999). However, recent studies showed that the 347Cys allele was associated with relatively lower hypertension prevalence in a Chinese population (Gu et al. 2006), and its carriers had a significantly greater blood pressure decrease with short-term irbesartan (angiotensin II type I receptor antagonist) treatment in Chinese hypertensive subjects (Jiang et al. 2005). These results suggested that genetic variations in ADRA1A could modulate cardiac or vascular sympathetic tone and might contribute to the pathogenesis of hypertension and cardiovascular disease.
Several polymorphisms have been described in the 5′ untranslated region (5′ UTR), coding region, and 3′ UTR of ADRA2A (Belfer et al. 2005; Kurnik et al. 2006; Small et al. 2006). The α2A-AR Asn251Lys polymorphism is located in the third cytoplasmic loop of the receptor and confers enhanced agonist-promoted Gi coupling (Small et al. 2000a). However, the 251Lys allele frequency was found to be relatively rare, e.g., 0.4% in Caucasian and 5% in African Americans (Small et al. 2003). DraI RFLP of 6.3- and 6.7-kb alleles has been found in the 3′ UTR of ADRA2A and has been reported to be associated with essential hypertension (Lockette et al. 1995), increased cardiovascular reactivity, body fat distribution (Oppert et al. 1995), platelet aggregation (Freeman et al. 1995), and glucose metabolism (Ukkola et al. 2000). The C-1291G polymorphism is located in the promoter region of ADRA2A. Previous studies have examined the associations between this polymorphism and various pathological states including hypertension, obesity phenotypes, and attention-deficit/hyperactivity disorder (Garenc et al. 2002; Rosmond et al. 2002; Wang et al. 2006); however, studies are still in the development stage.
A polymorphism consisting of the deletion of four consecutive amino acids (at position 322–325) has been identified in the α2C-AR. This variant receptor (α2CDel322–325) is associated with decreased Gi-protein coupling and impairment in the subsequent signaling pathway, which can enhance norepinephrine release, as compared with the wild-type (WT) receptor (Small et al. 2000b). Human studies have confirmed the contribution of α2CDel322–325 to an increased catecholamine release or catecholaminergic function (Gerson et al. 2003; Neumeister et al. 2005). The α2CDel322–325 polymorphism is associated with an increased risk of heart failure in African Americans (Small et al. 2002) and increased severity of heart failure symptoms (Brede et al. 2002; Small et al. 2002).
The unanswered question is whether physiological significance, especially cardiac sympathetic function, can be attributed to these α-AR polymorphisms, rather than to a pre-existing disease. We recently reported the association between the α2B-AR insertion/deletion polymorphism and autonomic nervous system (ANS) function in young healthy subjects by power spectral analysis of heart rate variability (HRV) (Suzuki et al. 2003; Yasuda et al. 2006).
For the same young and healthy study population, we investigated in the present study the association between the five representative α-AR polymorphisms (Arg347Cys of α1A-AR; C-1291G, Asn251Lys, and DraI RFLP of α2A-AR; and Del322–325 of α2C-AR) and ANS function. ANS function was assessed by analysis of HRV (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology 1996), which provides useful information on cardiac autonomic modulation (Akselrod et al. 1981; Pumprla et al. 2002) and is an early and sensitive indicator of pathologic states of hypertension or CVD (Liao et al. 1996, 1997). As described in previous reports, power spectral analysis of HRV has generally shown two major distinct regions of periodicity in electrocardiogram (ECG) R–R intervals: a high-frequency (HF) component (>0.15 Hz) and a low-frequency (LF) component (<0.15 Hz). Previous studies have showed that the HF component is mediated solely by parasympathetic nervous system activity, and that the LF component arises from both sympathetic and parasympathetic nervous activities (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology 1996; Pumprla et al. 2002). In addition, the ratio of LF to HF component has been considered as an index of sympatho-vagal balance or sympathetic activity (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology 1996; Piccirillo et al. 1996; Pumprla et al. 2002).
Methods
Study population
One hundred forty-nine young healthy Japanese males, recruited at random from Kyoto University, participated in each examination after written informed consent was obtained. Of these, 93 were subjects in our previous report (Suzuki et al. 2003), and 56 subjects were newly recruited in the present study. The ages of subjects ranged from 18–31 years (21.3±0.2, mean±SEM). All subjects were normotensive (causal supine BP<140/90 mmHg) and nonobese [body mass index (BMI)<30 kg/m2]. It was determined by interview that they were not taking any medication and had no history of organic diseases such as CVD, metabolic disorder, renal disease, or neuropathy. BMI, BP (systolic and diastolic BP), and heart rate (supine rest/standing) were measured as baseline characteristics, and family history (including whether or not subjects had relatives within the third degree who had hypertension, diabetes, or were obese) were investigated through interviews. All subjects underwent ECG recording and power spectral analysis of HRV. However, HRV in the standing position could not be determined for eight subjects.
The study protocol was reviewed by the appropriate institutional review committee of Kyoto University School of Medicine, and the guidelines of the Declaration of Helsinki were followed.
Genotyping
Genomic DNA was extracted from whole blood (DNA Extractor WB Kit, Wako, Osaka, Japan). Polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP)-based techniques were applied to genotype the study subjects for Arg347Cys polymorphism of α1A-AR; C-1291G, Asn251Lys polymorphisms, and DraI RLFP of α2A-AR; and a deletion polymorphism (Del322–325) of α2C-AR, as previously described (Garenc et al. 2002; Park et al. 2005; Shibata et al. 1996; Small et al. 2000a, b). The genotype of the α2C-AR Del322–325 polymorphism of one subject could not be determined because of an inadequate blood sample.
ECG R–R interval power spectral analysis
For details of the HRV analysis methodology, refer to the reviews (Tastk Force 1996; Akselrod et al. 1981; Pumprla et al. 2002). Each subject was studied in a quiet room with an ambient temperature of 25°C. They rested supine for at least 20 min before ECG recording. The CM5 lead ECG was continuously recorded during supine rest and postural change to a standing position. After 10 min of supine rest measurement, the subjects stood up by the bedside and remained at standing rest for another 10 min. During the test, the respiratory rate was controlled at 0.25 Hz (15 breaths/min) by means of an electric metronome to reduce significant variations in HRV spectral powers resulting from individual variability in breathing frequency and to avoid interference with the LF component by the parasympathetic component (Brown et al. 1993).
The R–R interval power spectral analysis procedures have been described previously (Matsunaga et al. 2005; Nishikino et al. 2006; Suzuki et al. 2003; Yasuda et al. 2006). Briefly, the ECG R–R interval data obtained from the CM5 lead were digitized at 1,000 Hz, and the derived R–R interval time series were then aligned in a 2-Hz sequence for power spectral analysis. The DC component and linear trend were completely eliminated by digital filtering for band-pass between 0.007 and 0.5 Hz. After passing through the Hamming-type data window, power spectral analysis was performed by means of a fast Fourier transform on the consecutive 480-s time series of R–R interval data obtained during the tests.
We evaluated very low frequency (VLF, 0.007–0.035 Hz), low frequency (LF, 0.035–0.15 Hz), high frequency (HF, 0.15–0.5 Hz), and total power (TP, 0.007–0.5 Hz) by integrating the spectrum for the respective bandwidth. The LF and HF powers were expressed in both absolute units (ms2) and in normalized units (%); normalized LF or HF powers were calculated as follows: LF(%)=(LF/TP−VLF)×100; HF(%)=(HF/TP−VLF)×100. The ratio of LF to HF (LF/HF) was also calculated. The average heart rate in beats per minute in each position (supine rest and standing) was derived from the R waves of the ECG.
Statistical analysis
Hardy–Weinberg equilibrium was verified by comparison of the observed and expected genotype frequencies using the χ2 test. Following the previous reports (Liao et al. 1996, 1997; Matsunaga et al. 2005; Piccirillo et al. 1996), a natural logarithmic transformation was used to normalize the distribution of HRV power spectral indices because these data showed a distribution skewed to the right. Differences in BMI, BP, and log-transformed values (ln) of HRV indices were evaluated by one-way ANOVA or Student’s t-test where appropriate. The data are expressed as mean±SEM. The χ2 test was performed for analysis of the relationship of genotype distributions to family history of hypertension, diabetes, or obesity. Statistical analysis was performed using the Statview Statistical Package (SAS Institute, Cary, NC, USA). Significant differences were considered to be present at P<0.05.
Results
Characteristics of the study subjects
Table 1 shows genotype and allele frequency for each α-AR polymorphism in the subjects of the present study. There was no detectable deviation from the Hardy–Weinberg equilibrium for α1A-AR Arg347Cys (χ 2=0.03, P=0.86), α2A-AR C-1291G (χ 2=0.22, P=0.64), and α2A-AR DraI (χ 2=0.09, P=0.76) polymorphisms, but there was for α2C-AR Del322–325 polymorphism (χ 2=4.37, P=0.04). α2A-AR Asn251Lys polymorphism was not detected. The allele frequencies of these polymorphisms were different for different ethnic backgrounds as shown in previous reports (Shibata et al. 1996; Small et al. 2004, 2006; Xie et al. 1999).
Table 2 shows the clinical characteristics for each α-AR polymorphism according to the genotype. Since the number of subjects homozygous for the α1A-AR 347Cys allele or α2C-AR Del322–325 allele was limited, statistical comparison was performed according to the presence or absence of the α1A-AR 347Cys allele or the α2C-AR Del322–325 allele. There were no significant differences in any of the clinical characteristics for all the studied polymorphisms.
Association of α-AR gene polymorphisms with HRV indices
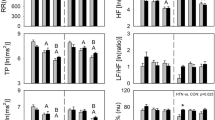
Table 3 shows the ECG R–R interval power spectral parameters according to α1A-AR Arg347Cys polymorphism. At supine rest, 347Cys allele carriers had a significantly higher HF(%) and lower LF(%) and LF/HF than Arg/Arg carriers. This result suggested that the α1A-AR 347Cys allele was associated with a relatively lower resting sympathetic activity accompanying higher parasympathetic activity.
No association was found between α2A-AR C-1291G genotype and HRV indices (Table 4). For the DraI RFLP, heterozygosity for the 6.3-kb allele showed a tendency to lower LF power and a significantly lower HF power, compared with 6.7-kb homozygote carriers (Table 4). However, significant differences in these indices were not observed in comparison with 6.3-kb homozygote carriers.
Marginal significance was obtained for the association between α2C-AR Del322–325 polymorphism and the HRV indices at supine rest (Table 5). α2CDel322–325 allele carriers had markedly higher HRV sympathetic indices [LF(%) and LF/HF] and lower HRV parasympathetic index [HF(%)] than noncarriers of this variant.
No significant difference was observed in any of the ECG R–R interval power spectral parameters for the standing position measurements for any of the α-AR polymorphisms.
Discussion
In the present study, using HRV power spectral analysis, we found that certain α-AR polymorphisms were significantly associated with cardiac ANS function in young and healthy Japanese men. In a supine rest, lower HRV sympathetic index [LF(%), LF/HF] and higher HRV parasympathetic index [HF(%)] were observed in α1A-AR 347Cys allele carriers than in Arg/Arg carriers, which suggested that the α1A-AR 347Cys allele was associated with potential attenuation of cardiac sympathetic tone accompanying vagal predominance. On the other hand, the α2C-AR Del322–325 allele carriers had higher sympathetic and lower parasympathetic activities as reflected in the HRV indices than noncarriers of this variant; this, however, was of marginal statistical significance. This observation suggested that the α2C-AR Del322–325 mutation might be associated with cardiac sympathetic predominance even in healthy normotensive states, which may shift sympatho-vagal valance toward vagal withdrawal. The subjects in the present study were young and healthy, therefore, the observed results suggest that the α1A-AR and α2C-AR polymorphisms contribute primarily to variability in cardiac sympatho-vagal modulation, which is not a secondary phenotype derived from hypertension or cardiovascular diseases.
Through sympathetic regulation, human α1A-AR response is associated with blood pressure homeostasis and cardiac development or function, and its dysfunction is associated with hypertension, benign prostatic hyperplasia, and cardiac hypertrophy (Guimaraes and Moura 2001; Tanoue et al. 2002). Several previous studies could not detect a significant association of the α1A-AR Arg347Cys variant with hypertension (Xie et al. 1999), benign prostate hyperplasia (Shibata et al. 1996), and agonist-induced vascular response (Sofowora et al. 2004).
In contrast with these studies, a recent study by Jiang et al. (2005) showed that α1A-AR 347Cys allele carriers had a significantly greater blood pressure response to short-term irbesartan treatment. Snapir et al. (2003) demonstrated that young healthy subjects homozygous for the 347Cys allele had a longer electrocardiogram (ECG) PR interval before and during the adrenaline infusion. Gu et al. (2006) showed that frequency of the 347Cys was significantly lower in a hypertensive group than in the control group, although they suggested other α1A-AR genetic variations or certain haplotypes also play an important role in the increased risk of essential hypertension. Observed association of the 347Cys allele with decreased cardiac sympathetic activity in the present study may be in part responsible for the (patho)physiological implications of this polymorphism. Thus, we could not exclude the possibility that the Arg347Cys polymorphism or other functional genetic variants in linkage disequilibrium with it may have potential clinical implications for α1A-AR-mediated physiology or pharmacology in relation to sympatho-vagal modulation (Buzas et al. 2004; Gu et al. 2006). Haplotype analysis may provide more information than analysis of single polymorphisms.
The α2C-AR subtype, as well as the α2A-AR, participated in a negative feed-back loop regulating norepinephrine release (Brede et al. 2003; Philipp et al. 2002). Studies in gene-targeted mice have demonstrated that disruption of α2C-AR causes increased circulating catecholamine levels, which will consequently lead to enhanced cardiac β-AR signaling, development of dilated cardiomyopathy, and increased mortality resulting from heart failure (Brede et al. 2002).
In humans, the α2C-AR Del322–325 polymorphism is related to augmented sympathetic and adrenomedullary activities even in healthy normotensive states (Neumeister et al. 2005). In line with previous observations, we found in the present study that the α2CDel322–325 allele carriers presented with sympathetic predominance as reflected in the HRV indices. In cardiovascular diseases including heart failure, sympathetic overactivation is closely involved in disease exacerbation and increased morbidity and mortality (Brook and Julius 2000), and it has been suggested that sympathetic predominance can also contribute to prospective pathogenesis of hypertension and CVD (Liao et al. 1996, 1997).
Some clinical studies have indicated that the α2CDel322–325 polymorphism is associated with an increased risk of heart failure in African Americans (Small et al. 2002) and increased severity of heart failure symptoms (Brede et al. 2002). However, ethnic variations in the frequencies of different haplotypes consisting of 20 α2C-AR polymorphisms (Small et al. 2004) may be important in the impact of the α2CDel322–325 variant on the pathology of cardiac disease. In addition, Small et al. (2002) reported the synergistic effect of the α2CDel322–325 and β1-AR Gly389Arg polymorphisms on the development of heart failure. Gene–gene interactions could modify the pathophysiological phenotypes of only one genetic variant.
α2A-AR regulates the release of catecholamine from sympathetic nerve terminals at the cardiovascular, central, and peripheral levels (Brede et al. 2003; Philipp et al. 2002). α2-AR agonist, clonidine, has been shown to reduce sympathetic activity and increase parasympathetic tone (Girgis et al. 1998; Tank et al. 2004) and has been used in clinical applications including blood pressure reduction and anesthesia.
Multiple genetic variations in ADRA2A have been identified to date (Belfer et al. 2005; Kurnik et al. 2006; Small et al. 2006). The 251Lys allele was found to be relatively rare in Caucasian and Asian populations (Small et al. 2006), and we also could not detect this polymorphism in 149 Japanese males (Table 1). Conversely, the 6.3-kb allele of the DraI RFLP and -1291G allele of ADRA2A were more common in Asians (Small et al. 2006), including a Japanese population (Table 1). The DraI RFLP has been reported to be associated with hypertension and increased autonomic responsiveness to some stressful conditions (Finley et al. 2004; Freeman et al. 1995; Lockette et al. 1995). In the present study, we found that heterozygous carriers of the 6.3-kb allele showed a lower HF and LF component power of HRV compared with 6.7-kb homozygous carriers; however, significant differences were not observed in comparison with 6.3-kb homozygous carriers. This may be due to the low number of these carriers (n=19). Large-scale study is required to detect the precise impacts of this polymorphism on ANS function.
Few studies have investigated an association of C-1291G polymorphism with hypertension-related phenotypes. One report showed that -1291C/G heterozygotes had significantly higher cortisol response to dexamethasone, fasting glucose, and tendency to increase diastolic blood pressure (Rosmond et al. 2002). In the present study, we could not detect a significant association of the C-1291G polymorphism with the HRV parameters in Japanese subjects. Linkage disequilibrium among the multiple ADRA2A polymorphisms could modulate the association with α2A-AR related diseases. In this respect, a recent study by Small et al. demonstrated that 16 single-nucleotide polymorphisms organized into 17 haplotypes were identified in ADRA2A, and its frequencies were markedly different among races, and these haplotypes lead to the diversity of α2A-AR receptor expressions (Small et al. 2006).
The present study has potential limitations. First, because the study subjects included only male Japanese, our conclusions cannot be generalized to other populations. In this context, the distribution patterns of the studied α-AR polymorphisms are different among various races (Shibata et al. 1996; Small et al. 2004, 2006; Xie et al. 1999). Second, the study subjects were young and healthy, therefore, present observations should be considered cautiously in relation to the pathology of hypertension or cardiovascular diseases and can not be applied to the phenotypes of patients with these diseases. Follow-up study is needed to elucidate the long-term effects of α-AR polymorphisms on autonomic function and to evaluate the prevalence of these diseases.
Third, we do not completely exclude the possibility that the results in the present study may be a false positive due to the multiple testing (five genetic markers and five HRV parameters). If the Bonferroni correction for multiple comparisons is applied (P<0.001 as significant level), the statistical results in this study will become insignificant. However, the α2A-AR C-1291G and DraI polymorphisms are in complete disequilibrium (|D′|=1.0, Haploview program), and each α-AR functionally interacts with the others in the heart. In addition, HRV indices were highly to moderately correlated with each other (data not shown). Therefore, in this case, each new test does not provide a completely independent opportunity for a type I error, and the Bonferroni correction for multiple testing will be too conservative (Nyholt 2001).
Rather than just presenting statistically significant results, in the present study, the simple original statistical results have been adopted in order to report some potentially important associations that are likely to be worth pursuing further. On the other hand, in order to reduce the number of statistical tests and the type-I error rate, we tried to perform multivariate ANOVA (MANOVA) analysis incorporating all of the studied α-AR polymorphisms (except for the α2A-AR Asn251Lys polymorphism, which was not found in the present subjects) and all five HRV parameters, in addition to univariate analyses. We could not find a significant association between α2C-AR Del322–325 polymorphism and HRV indices in MANOVA (P=0.605), which indicated that the results of the univariate analysis of α2C-AR Del322–325 should be interpreted with caution, taking into account the possibility of a sampling bias in this study population. Meanwhile, a significant association between the α1A-AR Arg347Cys polymorphism and HRV indices in the supine position was detected by both MANOVA (P=0.028) and univariate analyses.
Fourth, in the present study, the genotype distribution of the α2C-AR Del322–325 polymorphism was not in equilibrium. Although the precise reason for the observed deviation from Hardy–Weinberg proportion is unclear, one possible explanation could be the small population size. However, the other studied α-AR polymorphisms were in perfect Hardy–Weinberg equilibrium. Whatever the cause, the deviation from equilibrium could weaken the association between the α2C-AR Del322–325 allele and ANS function in this study.
Finally, the findings in the present study should be considered as preliminary because several other polymorphisms have been identified in each α-AR (Buzas et al. 2004; Gu et al. 2006; Small et al. 2004, 2006), and therefore, the haplotype or gene–gene interactions may modulate the association of α-AR polymorphisms with cardiac autonomic function. Analyses stratified by the haplotypes or combined genotypes for the α-AR polymorphisms are required in a large number of subjects.
In conclusion, the present study showed that certain α-AR genetic variations actually influence cardiac ANS function in young and healthy subjects, although these observations should be confirmed by a large-scale study in the same ethnic population. Abnormality of autonomic function is closely related to the pathophysiology of metabolic and vascular diseases, and can be a predictor of future incidence of these diseases. This leads us to hypothesize that, through autonomic modulation, certain α-AR polymorphisms might provide potential risks for various α-AR-related diseases. Short-term HRV analysis used in the present study is a highly reproducible method (Tarkiainen et al. 2005) and is of significant clinical relevance (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology 1996; Pumprla et al. 2002). Therefore, HRV can be used to search for genetic variation influencing autonomic modulation and various related diseases including cardiac disease (Kupper et al. 2004). When each α1-AR or α2-AR subtype-selective agent in humans is available, our understanding of the physiological and clinical importance of the α-AR polymorphisms will be further refined.
References
Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ (1981) Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 213:220–222
Belfer I, Buzas B, Hipp H, Phillips G, Taubman J, Lorincz I, Evans C, Lipsky RH, Enoch MA, Max MB, Goldman D (2005) Haplotype-based analysis of alpha 2A, 2B, and 2C adrenergic receptor genes captures information on common functional loci at each gene. J Hum Genet 50:12–20
Brede M, Wiesmann F, Jahns R, Hadamek K, Arnolt C, Neubauer S, Lohse MJ, Hein L (2002) Feedback inhibition of catecholamine release by two different alpha2-adrenoceptor subtypes prevents progression of heart failure. Circulation 106:2491–2496
Brede M, Nagy G, Philipp M, Sorensen JB, Lohse MJ, Hein L (2003) Differential control of adrenal and sympathetic catecholamine release by alpha 2-adrenoceptor subtypes. Mol Endocrinol 17:1640–1646
Brook RD, Julius S (2000) Autonomic imbalance, hypertension, and cardiovascular risk. Am J Hypertens 13:112S–122S
Brown TE, Beightol LA, Koh J, Eckberg DL (1993) Important influence of respiration on human R–R interval power spectra is largely ignored. J Appl Physiol 75:2310–2317
Buzas B, Belfer I, Hipp H, Lorincz I, Evans C, Phillips G, Taubman J, Max MB, Goldman D (2004) Haplotype block and superblock structures of the alpha1-adrenergic receptor genes reveal echoes from the chromosomal past. Mol Genet Genomics 272:519–529
Dao TT, Kailasam MT, Parmer RJ, Le HV, Le Verge R, Kennedy BP, Ziegler G, Insel PA, Wright FA, O’Connor DT (1998) Expression of altered alpha2-adrenergic phenotypic traits in normotensive humans at genetic risk of hereditary (essential) hypertension. J Hypertens 16:779–792
Eichler HG, Ford GA, Blaschke TF, Swislocki A, Hoffman BB (1989) Responsiveness of superficial hand veins to phenylephrine in essential hypertension alpha adrenergic blockade during prazosin therapy. J Clin Invest 83:108–112
Finley JC Jr, O’Leary M, Wester D, MacKenzie S, Shepard N, Farrow S, Lockette W (2004) A genetic polymorphism of the alpha2-adrenergic receptor increases autonomic responses to stress. J Appl Physiol 96:2231–2239
Freeman K, Farrow S, Schmaier A, Freedman R, Schork T, Lockette W (1995) Genetic polymorphism of the alpha 2-adrenergic receptor is associated with increased platelet aggregation, baroreceptor sensitivity, and salt excretion in normotensive humans. Am J Hypertens 8:863–869
Garenc C, Perusse L, Chagnon YC, Rankinen T, Gagnon J, Borecki IB, Leon AS, Skinner JS, Wilmore JH, Rao DC, Bouchard C (2002) The alpha 2-adrenergic receptor gene and body fat content and distribution: the HERITAGE Family Study. Mol Med 8:88–94
Gerson MC, Wagoner LE, McGuire N, Liggett SB (2003) Activity of the uptake-1 norepinephrine transporter as measured by I-123 MIBG in heart failure patients with a loss-of-function polymorphism of the presynaptic alpha2C-adrenergic receptor. J Nucl Cardiol 10:583–589
Girgis I, Chakko S, de Marchena E, Jara C, Diaz P, Castellanos A, Myerburg RJ (1998) Effect of clonidine on heart rate variability in congestive heart failure. Am J Cardiol 82:335–337
Gu D, Ge D, Snieder H, He J, Chen S, Huang J, Li B, Chen R, Qiang B (2006) Association of alpha1A adrenergic receptor gene variants on chromosome 8p21 with human stage 2 hypertension. J Hypertens 24:1049–1056
Guimaraes S, Moura D (2001) Vascular adrenoceptors: an update. Pharmacol Rev 53:319–356
Jiang S, Mao G, Zhang S, Hong X, Tang G, Li Z, Liu X, Zhang Y, Wang B, Xu X, Wang X (2005) Individual and joint association of alpha1A-adrenergic receptor Arg347Cys polymorphism and plasma irbesartan concentration with blood pressure therapeutic response in Chinese hypertensive subjects. Clin Pharmacol Ther 78:239–248
Kupper NH, Willemsen G, van den Berg M, de Boer D, Posthuma D, Boomsma DI, de Geus EJ (2004) Heritability of ambulatory heart rate variability. Circulation 110:2792–2796
Kurnik D, Muszkat M, Li C, Sofowora GG, Solus J, Xie HG, Harris PA, Jiang L, McMunn C, Ihrie P, Dawson EP, Williams SM, Wood AJ, Stein CM (2006) Variations in the alpha2A-adrenergic receptor gene and their functional effects. Clin Pharmacol Ther 79:173–185
Lafontan M, Berlan M (1995) Fat cell alpha 2-adrenoceptors: the regulation of fat cell function and lipolysis. Endocr Rev 16:716–738
Liao D, Cai J, Barnes RW, Tyroler HA, Rautaharju P, Holme I, Heiss G (1996) Association of cardiac autonomic function and the development of hypertension: the ARIC study. Am J Hypertens 9:1147–1156
Liao D, Cai J, Rosamond WD, Barnes RW, Hutchinson RG, Whitsel EA, Rautaharju P, Heiss G (1997) Cardiac autonomic function and incident coronary heart disease: a population-based case-cohort study. The ARIC Study. Atherosclerosis Risk in Communities Study. Am J Epidemiol 145:696–706
Lockette W, Ghosh S, Farrow S, MacKenzie S, Baker S, Miles P, Schork A, Cadaret L (1995) Alpha 2-adrenergic receptor gene polymorphism and hypertension in blacks. Am J Hypertens 8:390–394
Masuki S, Eisenach JH, Dinenno FA, Joyner MJ (2006) Reduced forearm alpha1-adrenergic vasoconstriction is associated with enhanced heart rate fluctuations in humans. J Appl Physiol 100:792–799
Matsunaga T, Nagasumi K, Yamamura T, Gu N, Nishikino M, Ueda Y, Moritani T, Aoki N, Tsuda K, Yasuda K (2005) Association of C825T polymorphism of G protein beta3 subunit with the autonomic nervous system in young healthy Japanese individuals. Am J Hypertens 18:523–529
Neumeister A, Charney DS, Belfer I, Geraci M, Holmes C, Sharabi Y, Alim T, Bonne O, Luckenbaugh DA, Manji H, Goldman D, Goldstein DS (2005) Sympathoneural and adrenomedullary functional effects of alpha2C-adrenoreceptor gene polymorphism in healthy humans. Pharmacogenet Genomics 15:143–149
Nishikino M, Matsunaga T, Yasuda K, Adachi T, Moritani T, Tsujimoto G, Tsuda K, Aoki N (2006) Genetic variation in the renin-angiotensin system and autonomic nervous system function in young healthy Japanese subjects. J Clin Endocrinol Metab (in press). DOI 10.1210/jc.2006-0700
Nyholt DR (2001) Genetic case-control association studies—correcting for multiple testing. Hum Genet 109:564–567
Oppert JM, Tourville J, Chagnon M, Mauriege P, Dionne FT, Perusse L, Bouchard C (1995) DNA polymorphisms in the alpha 2- and beta 2-adrenoceptor genes and regional fat distribution in humans: association and linkage studies. Obes Res 3:249–255
Park L, Nigg JT, Waldman ID, Nummy KA, Huang-Pollock C, Rappley M, Friderici KH (2005) Association and linkage of alpha-2A adrenergic receptor gene polymorphisms with childhood ADHD. Mol Psychiatry 10:572–580
Philipp M, Brede M, Hein L (2002) Physiological significance of alpha (2)-adrenergic receptor subtype diversity: one receptor is not enough. Am J Physiol Regul Integr Comp Physiol 283:R287–R295
Piccirillo G, Munizzi MR, Fimognari FL, Marigliano V (1996) Heart rate variability in hypertensive subjects. Int J Cardiol 53:291–298
Pumprla J, Howorka K, Groves D, Chester M, Nolan J (2002) Functional assessment of heart rate variability: physiological basis and practical applications. Int J Cardiol 84:1–14
Rosmond R, Bouchard C, Bjorntorp P (2002) A C-1291G polymorphism in the alpha2A-adrenergic receptor gene (ADRA2A) promoter is associated with cortisol escape from dexamethasone and elevated glucose levels. J Intern Med 251:252–257
Shibata K, Hirasawa A, Moriyama N, Kawabe K, Ogawa S, Tsujimoto G (1996) Alpha 1a-adrenoceptor polymorphism: pharmacological characterization and association with benign prostatic hypertrophy. Br J Pharmacol 118:1403–1408
Small KM, Forbes SL, Brown KM, Liggett SB (2000a) An Asn to Lys polymorphism in the third intracellular loop of the human alpha 2A-adrenergic receptor imparts enhanced agonist-promoted Gi coupling. J Biol Chem 275:38518–38523
Small KM, Forbes SL, Rahman FF, Bridges KM, Liggett SB (2000b) A four amino acid deletion polymorphism in the third intracellular loop of the human alpha 2C-adrenergic receptor confers impaired coupling to multiple effectors. J Biol Chem 275:23059–23064
Small KM, Wagoner LE, Levin AM, Kardia SL, Liggett SB (2002) Synergistic polymorphisms of beta1- and alpha2C-adrenergic receptors and the risk of congestive heart failure. N Engl J Med 347:1135–1142
Small KM, McGraw DW, Liggett SB (2003) Pharmacology and physiology of human adrenergic receptor polymorphisms. Annu Rev Pharmacol Toxicol 43:381–411
Small KM, Mialet-Perez J, Seman CA, Theiss CT, Brown KM, Liggett SB (2004) Polymorphisms of cardiac presynaptic alpha2C adrenergic receptors: diverse intragenic variability with haplotype-specific functional effects. Proc Natl Acad Sci USA 101:13020–13025
Small KM, Brown KM, Seman CA, Theiss CT, Liggett SB (2006) Complex haplotypes derived from noncoding polymorphisms of the intronless alpha2A-adrenergic gene diversify receptor expression. Proc Natl Acad Sci USA 103:5472–5477
Snapir A, Koskenvuo J, Toikka J, Orho-Melander M, Hinkka S, Saraste M, Hartiala J, Scheinin M (2003) Effects of common polymorphisms in the alpha1A-, alpha2B-, beta1- and beta2-adrenoreceptors on haemodynamic responses to adrenaline. Clin Sci (Lond) 104:509–520
Sofowora GG, Dishy V, Landau R, Xie HG, Prasad HC, Byrne DW, Smiley RM, Kim RB, Wood AJ, Stein CM (2004) Alpha 1A-adrenergic receptor polymorphism and vascular response. Clin Pharmacol Ther 75:539–545
Suzuki N, Matsunaga T, Nagasumi K, Yamamura T, Shihara N, Moritani T, Ue H, Fukushima M, Tamon A, Seino Y, Tsuda K, Yasuda K (2003) Alpha(2B)-adrenergic receptor deletion polymorphism associates with autonomic nervous system activity in young healthy Japanese. J Clin Endocrinol Metab 88:1184–1187
Tank J, Diedrich A, Szczech E, Luft FC, Jordan J (2004) Alpha-2 adrenergic transmission and human baroreflex regulation. Hypertension 43:1035–1041
Tanoue A, Koshimizu TA, Tsujimoto G (2002) Transgenic studies of alpha(1)-adrenergic receptor subtype function. Life Sci 71:2207–2215
Tarkiainen TH, Timonen KL, Tiittanen P, Hartikainen JE, Pekkanen J, Hoek G, Ibald-Mulli A, Vanninen EJ (2005) Stability over time of short-term heart rate variability. Clin Auton Res 15:394–399
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996) Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93:1043–1065
Ukkola O, Rankinen T, Weisnagel SJ, Sun G, Perusse L, Chagnon YC, Despres JP, Bouchard C (2000) Interactions among the alpha2-, beta2-, and beta3-adrenergic receptor genes and obesity-related phenotypes in the Quebec Family Study. Metabolism 49:1063–1070
Wang B, Wang Y, Zhou R, Li J, Qian Q, Yang L, Guan L, Faraone SV (2006) Possible association of the alpha-2A adrenergic receptor gene (ADRA2A) with symptoms of attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet 141:130–134
Xie HG, Kim RB, Stein CM, Gainer JV, Brown NJ, Wood AJ (1999) Alpha1A-adrenergic receptor polymorphism: association with ethnicity but not essential hypertension. Pharmacogenetics 9:651–656
Yasuda K, Matsunaga T, Adachi T, Aoki N, Tsujimoto G, Tsuda K (2006) Adrenergic receptor polymorphisms and autonomic nervous system function in human obesity. Trends Endocrinol Metab 17:269–275
Acknowledgements
This study was supported by a grant provided by the Ministry of Education, Culture, Sports, Science and Technology, Japan, as part of the 21st century COE program “Knowledge Information Infrastructure for Genome Science.”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsunaga, T., Yasuda, K., Adachi, T. et al. Alpha-adrenoceptor gene variants and autonomic nervous system function in a young healthy Japanese population. J Hum Genet 52, 28 (2007). https://doi.org/10.1007/s10038-006-0076-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10038-006-0076-3