Abstract
Objectives
To analyse the value of cardiovascular magnetic resonance (CMR)-derived myocardial parameters to differentiate left ventricular non-compaction cardiomyopathy (LVNC) from other cardiomyopathies and controls.
Methods
We retrospectively analysed 12 patients with LVNC, 11 with dilated and 10 with hypertrophic cardiomyopathy and compared them to 24 controls. LVNC patients had to fulfil standard echocardiographic criteria as well as additional clinical and imaging criteria. Cine steady-state free precession and late gadolinium enhancement (LGE) imaging was performed. The total LV myocardial mass index (LV-MMI), compacted (LV-MMIcompacted), non-compacted (LV-MMInon-compacted), percentage LV-MMnon-compacted, ventricular volumes and function were calculated. Data were compared using analysis of variance and Dunnett’s test. Additionally, semi-quantitative segmental analyses of the occurrence of increased trabeculation were performed.
Results
Total LV-MMInon-compacted and percentage LV-MMnon-compacted were discriminators between patients with LVCN, healthy controls and those with other cardiomyopathies with cut-offs of 15 g/m2 and 25 %, respectively. Furthermore, trabeculation in basal segments and a ratio of non-compacted/compacted myocardium of ≥3:1 were criteria for LVNC. A combination of these criteria provided sensitivities and specificities of up to 100 %. None of the LVNC patients demonstrated LGE.
Conclusions
Absolute CMR quantification of the LV-MMInon-compacted or the percentage LV-MMnon-compacted and increased trabeculation in basal segments allows one to reliably diagnose LVNC and to differentiate it from other cardiomyopathies.
Key Points
• Cardiac magnetic resonance imaging can reliably diagnose left ventricular non-compaction cardiomyopathy.
• Differentiation of LVNC from other cardiomyopathies and normal hearts is possible.
• The best diagnostic performance can be achieved if combined MRI criteria for the diagnosis are used.
Similar content being viewed by others
Introduction
Left ventricular non-compaction (LVNC) is a rare cardiomyopathy characterised by numerous excessively prominent left ventricular (LV) trabeculation and deep intertrabecular recesses communicating with the ventricular cavity and severely altering myocardial structure. [1–3]. Although most authors assume a developmental arrest in embryogenesis as the underlying pathology [4, 5], the mechanisms of LVNC are not fully understood yet. Several gene mutations have been identified to be linked with LVNC and an autosomal dominant inheritance mode is frequent [6–9].
To date the most commonly used imaging tool for diagnosing LVNC is echocardiography applying the criteria established by Jenni and co-authors [2]. However, qualitative or even semi-quantitative parameters to differentiate normal compaction of the myocardium in healthy subjects from LVNC or from other cardiomyopathies like dilated cardiomyopathy (DCM) or hypertrophic cardiomyopathy (HCM) may fail because of highly variable LV trabeculation. Therefore, absolute quantification should be performed. Cardiovascular magnetic resonance (CMR) has been reported as a promising imaging modality to characterise patients with LVNC as it provides both a high spatial resolution and a good contrast between trabeculation and blood pool [10, 11]. Jacquier et al. recently reported that a value of non-compacted LV myocardial mass above 20 % of the global mass of the LV is highly sensitive and specific for LVNC [12]. However, in their approach, a substantial degree of the LV cavity was included in the calculated trabecular LV mass and led to systemic overestimation of the latter. Furthermore, no late gadolinium enhancement (LGE) imaging was performed in their work, which has been described as a potential prognostic factor in LVNC patients [13].
The aim of our retrospective study was to present improved quantitative CMR criteria to distinguish LVNC from DCM, HCM and a group of healthy controls, to add new qualitative and semi-quantitative criteria, and to analyse the occurrence of LGE in LVNC. We hypothesise that CMR can be used to diagnose LVNC with high sensitivity and specificity and can further help to differentiate LVNC from DCM and HCM.
Materials and methods
This overall retrospective study was approved by the local ethics committee and complies with the Declaration of Helsinki. All patients or parents gave written informed consent for use of their anonymised data. Data of the control group were assessed prospectively with a separate ethics committee approval. None of the authors received funding.
Patient population, control group and study design
Within a period of 60 months, 12 patients (3 male, 27 %) with LVNC were included in the study. These patients had to fulfil the echocardiographic criteria of Jenni [2]. Furthermore, to increase the probability of the diagnosis LVNC, these criteria had to be accompanied by the following additional clinical and imaging findings as described before [11]: (1) suspicion or confirmed diagnosis of LVNC in first degree relatives, (2) associated neuromuscular disorders and (3) complications such as systemic embolisation and/or regional wall motion abnormalities or ventricular tachycardias with or without syncopal attacks. A summary of the additional findings is given in Table 1. Exclusion criteria were an associated congenital or acquired heart disease and usual CMR contraindications such as implanted defibrillators/pacemakers.
Of the 12 included patients 8 initially presented with symptoms of heart failure. Three patients came for diagnostic evaluation of tachycardias, which were associated with syncopes in two patients. Of two patients with a familial anamnesis, one was clinically asymptomatic and came for screening.
CMR was performed within 3 days after echocardiography. None of the patients fulfilled exclusion criteria for CMR such as implanted defibrillators/pacemakers or intracranial metallic implants.
We furthermore retrospectively included 10 consecutive patients (4 male, 36 %) with HCM and 11 consecutive patients (3 male, 27 %) with DCM that underwent CMR within a period of 6 months. The diagnosis of HCM and DCM was established on clinical, echocardiographic and electrocardiographic criteria [14].
Patient parameters were compared to a gender-balanced control group of 24 healthy volunteers without history of cardiovascular disease and without clinical symptoms. Data of the control group were assessed prospectively and separately from the acquisition of the patient data covered by a separate ethics committee approval.
Patient and control group characteristics are presented in Table 2.
Genetic analysis
Two patients with familial LVNC underwent genetic analyses. Pedigree analyses indicated an autosomal dominant mode of inheritance. Exonic regions of six sarcomeric genes were analysed by single-stranded conformational polymorphism, denaturing gradient gel electrophoresis or direct cycle sequencing as previously described [7, 15]. The following genes were analysed in the respective index patients: troponin T, myosin binding protein C, alpha and beta myosin heavy chain, lamin A/C and phospholamban.
Cardiovascular magnetic resonance
All CMR examinations were performed on a 1.5-T scanner (Intera CV, Philips Healthcare, Best, the Netherlands) in supine position using a dedicated 5-channel phased array surface cardiac coil. Black-blood images with a slice thickness of 8 mm in axial orientation were acquired to visualize morphology. For volumetric and functional imaging, breath hold standard cine steady-state free precession sequences in short-axis 4-chamber view and LV vertical long-axis orientation were acquired covering the whole heart gapless from the apex to the base with a temporal resolution of 46 ms. Echo time was 1.8 ms, repetition time 3.6 ms, flip angle 50°, typical in-plane resolution was 1.7 × 1.8 mm, slice thickness 8 mm. LGE images in short-axis orientation were acquired for quantification of fibrosis 10–15 min after application of 0.2 mmol/kg/body weight gadopentetate dimeglumine (Magnevist, Bayer HealthCare Pharmaceuticals, Berlin, Germany) using a three-dimensional T1-weighted inversion recovery turbo gradient echo sequence. Echo time was 1.5 ms, repetition time 2.8 ms, flip angle 15°, typical in-plane resolution 1.8 × 2 mm, slice thickness 8 mm.
Image analysis
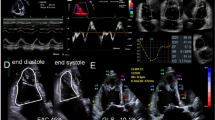
CMR image analysis was performed by two fully blinded observers, M.Gr. with 13 years and M.P. with 5 years of experience in CMR, on a standard personal computer in a random order in our CMR laboratory, which has expertise in imaging of congenital heart disease and has proven low intra- and interobserver variability for the assessment of cardiac biventricular volumes and function [16, 17]. The CAAS MRV software (Version 3, Pie-Medical Imaging, Maastricht, Netherlands) was used for contouring compacted and non-compacted myocardium. The algorithm has been previously described in detail [18]. In brief, LV epicardial borders were manually traced in end-systole and end-diastole in 4-chamber view and vertical long-axis view orientation. Registration marks were set at the level of the mitral valve and the apex. For LV segmentation a contour propagation algorithm based on the concept of fuzzy objects and “hanging togetherness” throughout the short-axis images was then performed [19]. In end-diastole, trabeculation that was not included in the automated segmentation was manually traced and marked with different colours than the compacted myocardium allowing for assessment of total LV myocardial mass index (LV-MMI), the compacted (LV-MMIcompacted), non-compacted (MMInon-compacted) and percentage LV-MMnon-compacted (Fig. 1a). During end-systole (Fig. 1b) trabeculation is usually compressed which limits the differentiation between non-compacted and compacted myocardium. For calculation of the myocardial mass the specific density of 1.05 g/ml was used. Additionally, LV end-diastolic volumes, end-systolic volumes, stroke volumes and ejection fractions were calculated. All volumetric measurements were performed twice by M.Gr. to measure intraobserver variability and once by M.P. to measure interobserver variability. Absolute parameters were normalized for body surface area [20].
a Assessment of non-compacted (pink contours) and compacted (red and blue contours) left ventricular myocardium using CAAS MRV software in controls, patients with left ventricular non-compaction cardiomyopathy (LVNC), dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy (HCM). Basal, midventricular and apical cine steady-state free precession images in short-axis orientation during end-diastole (echo time 1.8 ms, repetition time 3.6 ms, flip angle 50°). All masses were assessed in end-diastole. b Midventricular steady-state free precession (SSFP) images in short-axis orientation in end-systole. In this phase trabeculation is compressed which makes it almost impossible to differentiate non-compacted from compacted myocardium. Therefore, the end-systolic phase is not suitable either for measuring the ratio of non-compacted/compacted myocardium or for quantifying the amount of non-compacted myocardium. Furthermore, this may also lead to an overestimation of LV ejection fractions (EF) in LVNC and HCM patients
We furthermore performed a semi-quantitative analysis of trabeculated segments (TS) according to the 17-segment model [21] for the occurrence of increased trabeculation of the LV. The degree of trabeculated myocardium was measured in end-diastole in short-axis orientation (segment 17 in the 4-chamber view orientation) and the ratio was calculated. To simplify the assessment of the segmental degree of non-compacted myocardium for practical reasons it was categorised into four grades:
-
No trabeculation
-
Non-compacted/compacted myocardium ratio of <2:1
-
Non-compacted/compacted myocardium ratio of ≥2:1 to <3:1
-
Non-compacted/compacted myocardium ratio of ≥3:1
To enable comparison with previously published data [11], we additionally used the cut-off value of non-compacted/compacted myocardium of >2.3:1 for categorisation and calculated the different statistical parameters including and excluding segment 17, which can usually not be assessed by echocardiography (Table 4).
Statistics
Categorical variables are expressed as number and percentage. Continuous variables are given as mean ± standard deviation according to normal distribution of data in the Kolmogorov–Smirnov test. For comparison of means between the LVNC group and the other groups a univariate analysis of variance (ANOVA) in combination with Dunnett’s test was performed. The categorical data of the segmental analysis were analysed using the chi-square test or Fisher’s exact test. The intraobserver variability was analysed by calculating the coefficient of variation. Receiver operating characteristics (ROC) curves were used to determine optimal cut-off values for distinguishing LVNC from DCM, HCM and controls. Sensitivities, specificities, positive and negative prognostic values were calculated for the criteria diagnosing LVNC. The tests were performed as two-sided at a significance level α = 0.05. For statistical analysis SPSS software version 16.0 (SPSS Inc., Chicago, Il, USA) was used.
Results
In one LVNC patient image quality was impaired in the cine sequence because of breathing artefacts and no contour detection could be performed. This patient was excluded from volumetric analysis but included in the analysis of TS. No adverse events occurred. Patient and control group demographic data are presented in Table 2. Distribution of patient and control group parameters is summarised in Table 3.
The LVNC group was significantly older than the control group and younger than the HCM group (Table 2). Mutational analysis did not identify mutations in the six sarcomere genes that were screened.
Volumetric parameters and myocardial mass
In LV volumetry, 5 of the remaining 11 LVNC patients had a normal LV-EF of above 55 % according to published reference values [22]. Only one LVNC patient presented with significant LV enlargement, which was associated with an impaired LV-EF of 30 %. Total LV-MMInon-compacted and percentage LV-MMnon-compacted were significantly increased in LVNC and are good discriminators between patients with LVNC and all other cardiomyopathies and controls (P < 0.001 for all, Table 3).
For diagnosing LVNC ROC curves revealed optimal cut-off values of 25 % for percentage LV-MM non-compacted (1st criterion) and 15 g/m2 total LV-MMI non-compacted (2nd criterion) (Fig. 2, Table 4). Intraobserver variability for assessment of myocardial masses and volumetric parameters using the CAAS software was low with a mean difference of 3.1 %. The coefficient of variation was less than 2 for all measurements with a mean value of 1.25 ± 3.4 %. Interobserver variability was higher with a mean difference of 5.7 % and a coefficient of variation of 4.2 ± 2.2.
Receiver operating characteristic (ROC) curves showing the sensitivity and specificity of left ventricular non-compacted myocardial mass index (a) and percentage non-compacted myocardial mass (b) for diagnosing left ventricular non-compaction cardiomyopathy. Both parameters demonstrate excellent AUC values of 0.99. Cut-off values of 15 g/m2 and 25 % afford comparable sensitivities of 91 % and specificities of up to 100 %
Late gadolinium enhancement (LGE)
None of the LVNC patients demonstrated intramyocardial LGE. In contrast, LGE was found in all other patients. Of 10 HCM patients 8 presented with a patchy enhancement of the interventricular septum. Two HCM patients showed patchy, partly confluent enhancement also in the anterior wall.
In DCM hyperenhancement was also located in the interventricular septum in most cases. Nine of 11 patients demonstrated a streaky midwall LGE in the interventricular septum. In 3 patients this enhancement extended to the inferior wall.
Analysis of trabeculated segments (TS)
The analysis of trabeculation patterns demonstrated the specifics of LVNC patients with regard to number and distribution of TS. LVNC patients demonstrated an average of 12.6 ± 1.6 TS ,whereas the number was much lower in HCM patients (6 ± 1.2, P < 0.001), DCM patients (4.5 ± 0.9, P < 0.001) and controls (5.0 ± 1.4, P < 0.001). Analysis of the TS distribution revealed that in the LVNC group each segment showed trabeculation at least once but not in the DCM, HCM and control groups (Fig. 3a). In particular, the basal and septal segments 4–6 were trabeculated more frequently in LVNC patients. Also the degree of trabeculation was higher compared with HCM, DCM and controls (Fig. 3a). Even in the basal segments 1–6, which showed only little trabeculation in any of the other patients or controls, we found some LV-MMInon-compacted /LV-MMIcompacted ratios of ≥ 3:1.
Percentage, distribution and degree of trabeculated segments of the left ventricular myocardium of the different patient cohorts and healthy controls. Segmentation according to the 17-segment model of the American Heart Association. Legend shows the ratio of non-compacted/compacted myocardium. The distribution of trabeculation according to the classification of the present study is shown in a. The distribution according to the previously published cut-off of >2.3:1 is shown in b. Note the exclusive occurrence of a ≥ 3:1 trabeculation in the basal segments in LVNC patients and the overlap with other diseases, especially HCM but also DCM in the apical segments
Therefore, a ratio of non-compacted/compacted myocardium of ≥3:1 (3rd criterion) in at least one of the other segments (1–3, 7–16) showed a very high sensitivity of 100 % and specificity of 93 % as well as a very high NPV of 100 % (Table 4). If segment 17 was included the specificity decreased to 73 %.
Furthermore, an LV-MMInon-compacted /LV-MMIcompacted ratio ≥2:1 in the basal segments 4–6 was highly specific for the diagnosis of LVNC (4th criterion).
Applying the recently published cut-off value of >2.3:1 [11] to our study cohort and excluding segment 17 we obtained a sensitivity of 100 % but specificity was only 80 %. When including segment 17, the specificity further decreases (Table 4).
Combined criteria
The best diagnostic performance, which means the best compromise between a high sensitivity and specificity as well as a high PPV and NPV, could be achieved if a combined criteria approach for the diagnosis of the four identified single MRI parameters was used, especially when using the criterion that at least three of the four identified MRI criteria had to be positive for LVNC (Table 4).
Discussion
There is no specific treatment for LVNC. Nevertheless, the early and precise diagnosis is mandatory to rule out other underlying diagnoses and to allow a timely start of standard heart failure and anticoagulation therapy which may prevent further complications.
Our study provides redefined and extended CMR criteria for diagnosing and discriminating LVNC from other cardiomyopathies. These four basic criteria are (Table 4)
-
1.
Percentage LV-MMnon-compacted >25 %
-
2.
Total LV-MMInon-compacted >15 g/m2
-
3.
Non-compacted/compacted myocardium ratio of ≥3:1 in at least one of the other segments (1–3, 7–16) excluding the apical segment 17
-
4.
Trabeculation in segments 4–6 ≥ 2:1 (non-compacted/compacted)
There are some studies using echocardiographic criteria for diagnosing LVNC [2, 5]. However, this approach is highly investigator-dependent, diagnosis is based on two-dimensional planes using semi-quantitative or qualitative criteria and specificity is low [23]. Two-dimensional CMR criteria derived from echocardiography [11, 24] offer a better visualization of all regions of the LV but suffer from the same fundamental limitations. One major advantage of CMR is the three-dimensional approach, which allows for imaging of the entire volume of the heart with lower investigator dependency and without limitations caused by a patient’s constitution. Our results demonstrated a good reproducibility with low intra- and interobserver variability. Jacquier et al. [12] were first to measure the total amount of trabeculation and to propose a cut-off value above which the diagnosis of LVNC is likely. However, in their study endocardial contours were traced along the tip of LV trabeculation towards the LV cavity and therefore intertrabecular blood pool was included in the trabeculated mass, especially in LVNC patients.
In the present study we tried to overcome these limitations by excluding blood pool from the assessment of LV-MMInon-compacted. Additionally the purpose of this study was to analyse the occurrence of LGE in LVNC.
We performed genetic analysis in two patients in which, however, mutation screening was negative. A recent work showed that the LVNC patients with mutations in sarcomere genes are phenotypically not distinct from those without a mutation [25]. A negative genetic analysis of six sarcomere genes, like in our study, does therefore not rule out the diagnosis of LVNC. There is a wide variety in the location of the gene mutation, which can make the effort required for genetic analysis unreasonably high. The pedigree analyses are more often helpful and indicated an autosomal dominant mode of inheritance in these two patients.
Mass and distribution of LV trabeculation
Compared with Jaquier et al. results [12], our study shows some discrepancies in regard to LV volumes and masses. LV-MMInon-compacted was lower in all patient groups and controls in the present study, which is most likely the result of the exclusion of blood pool. However, percentage LV-MMnon-compacted of LVNC patients was similar, meaning that LV-MMIcompacted was lower in our group. This might be explained by the female predominance in our LVNC cohort (Table 2). In the same group we also found a lower LV-EDVI; however, our group was younger and presented with an LV-EF within a lower normal range (Table 3) compared with the clearly impaired LV function of the LVNC cohort of the cited study. The aforementioned differences result in a minor discrepancy of the percentage LV-MMnon-compacted (25 % vs. 20 %). Nevertheless, both studies clearly demonstrate that it is possible to diagnose LVNC using this parameter. The distinct assessment of LV trabeculation in the present study additionally allows for introduction of a total LV-MMInon-compacted cut-off value of 15 g/m2 (Fig. 4), making it is possible to diagnose LVNC independently of the mass of the compacted myocardium.
According to literature data, the distribution of TS in LVNC is controversial. In a large echocardiographic study with 34 patients the authors found TS in the midventricular and apical segments [26]. Jacquier et al. did not find a specific distribution of TS in LVNC patients compared to DCM, HCM and controls [12]. In the present study we could demonstrate that trabeculation in the segments 4–6 (Fig. 5) alludes to a high probability for LVNC (Fig. 3a). Only one HCM patient also showed trabeculation in these segments with a ratio of non-compacted/compacted myocardium of <2:1. As demonstrated in Fig. 3a the degree of trabeculation can also be considered as a good discriminator between LVNC and the other patient cohorts and controls. In the LVNC cohort the number of segments with a non-compacted/compacted myocardium ratio of ≥3:1 is highest by far not only in the basal and midventricular but also in the apical segments. The use of a myocardial compacted/non-compacted ratio is a common criterion for diagnosis of LVNC in both echocardiographic and CMR studies. However, there is controversy regarding the ratio value and whether it should be measured in end-systole or end-diastole. Petersen et al. considered a CMR ratio between the non-compacted and the compacted layer of >2.3:1 diagnostic for LVNC [11]. Applying this ratio to our study cohort (Fig. 3b) we achieved sensitivities of 100 % and specificities of 80 % (excluding segment 17) or 58 % (including segment 17). The specificities were much lower than in the cited study and also lower as compared to our single or combined criteria we propose (Table 4). Furthermore, the PPV was only 57 % and 39 % respectively. In the present study we found many HCM and DCM patients with a non-compacted/compacted ratio between 2:1 and 3:1. Of these some had a ratio of >2.3:1. These patients impair the specificity of the cited cut-off value as too many HCM and DCM patients have false positive results for LVNC.
The discrepancy between the study results could be caused by a different approach in the measurement of the thickness of compacted and non-compacted myocardium. Petersen et al. [11] used three long-axis views, whereas in our study short-axis views were used (except for segment 17).
A second possible explanation might be differences in the HCM and DCM patient cohorts; however, no details about these patients are provided in Petersen et al.’s report [11].
LVNC and LGE
None of our LVNC patients presented with myocardial LGE (Fig. 6). Our findings are in line with other studies that also stated a lack of LGE in LVNC [27, 28]. On the other hand, there are also studies that do report LGE findings [13, 29–31].
Late gadolinium enhancement (LGE) images (a–c) with typical enhancement patterns of hypertrophic cardiomyopathy (HCM) and dilated cardiomyopathy (DCM). Steady-state free precession (SSFP) images during end-systole (d–f) and end-diastole (g–i) of a typical left ventricular non-compaction (LVNC), HCM and DCM patient
The reasons for these discrepancies remain unclear. Firstly, the presence or absence of LGE does probably not depend on patient age as the mean age of our LVNC cohort without LGE (35 years) is framed by the mean ages of two patient groups with positive LGE [13, 31]. Secondly, there was no significant difference in the mean LV ejection fraction between our LVNC patients and the patients of one study [13]. Finally all cited imaging studies were performed using 1.5-T MR systems. However, the authors of the largest cited study [13] used a T1-weighted inversion recovery gradient echo sequence, depending on correct adjustments of inversion time [32], whereas a smaller study without positive LGE findings in LVNC patients used a more modern phase-sensitive inversion recovery sequence, which has shown advantages in visualisation of fibrosis in regard to image quality and reproducibility compared with standard magnitude detection [33]. Therefore, the contradictory LGE findings in the present and the cited studies might be partly caused by differences in imaging techniques.
The described macroscopic LGE patterns in LVNC patients varied substantially from subendocardial, transmural, intramyocardial to subendocardial [13, 30] and no specific or at least typical enhancement patterns for LVNC patients have been described so far. Therefore, especially in the adult group, an additional underlying coronary artery disease or e.g. postinflammatory changes causing different patterns of LGE described in LVNC patients have to be excluded to be sure that the macroscopic LGE is indeed an intrinsic finding of LVNC and not an epiphenomenon caused by other coexisting diseases.
The absence of LGE in our study cohort better corresponds with the theory of a developmental arrest [4, 5] as the underlying pathophysiology of LVNC than with the competing theory of a traumatic or ischemic pathophysiology [34]. According to the latter theory one would expect scar tissue and typical patterns of LGE in each LVNC patient. In developmental arrest though, caused by a genetic mutation, the absence of such alterations would not be contradictory. However, this might be a premature conclusion as the LGE technique might just overlook some cases of fibrosis as a result of the given spatial resolution.
The presence, pathophysiology and meaning of LGE in LVNC patients need to be confirmed by further studies with a genetic diagnosis and a larger sample size to increase statistical power and to potentially identify risk factors for developing fibrosis in LVNC.
Limitations
Genetic analysis was performed only in two LVNC patients with familial disease. However, no gene mutation could be identified indicating that other genes may be involved in the genetic aetiology of LVNC. A cohort of LVNC patients with a genetic diagnosis would increase the reliability of a CMR-derived diagnosis.
Owing to the prevalence of LVNC the study population was small in terms of statistical means. Multicentre studies are necessary to confirm our findings in a larger patient cohort.
Furthermore, CMR follow-up examinations would be helpful to assess a potential change of non-compacted or compacted mass in a chronological sequence.
In conclusion, CMR can distinguish LVNC from other cardiomyopathies and normal hearts with high sensitivity and specificity. In our study we introduce highly reproducible volumetric cut-off values and two semi-quantitative criteria. The role of the absence or presence of LGE in LVNC has to be further investigated.
Abbreviations
- CI:
-
confidence interval
- CMR:
-
cardiovascular magnetic resonance
- DCM:
-
dilated cardiomyopathy
- HCM:
-
hypertrophic cardiomyopathy
- LGE:
-
late gadolinium enhancement
- LV:
-
left ventricle/left ventricular
- LV-MMI:
-
left ventricular myocardial mass index
- LVNC:
-
left ventricular non-compaction
- TS:
-
trabeculated segment/s
References
Breckenridge RA, Anderson RH, Elliott PM (2007) Isolated left ventricular non-compaction: the case for abnormal myocardial development. Cardiol Young 17:124–9
Jenni R, Oechslin E, Schneider J, Attenhofer Jost CH, Kaufmann PA (2001) Echocardiographic and pathoanatomical characteristics of isolated LV non-compaction: a step towards classification as a distinct cardiomyopathy. Heart 86:666–71
Weiford BC, Subbarao VD, Mulhern KM (2004) Noncompaction of the ventricular myocardium. Circulation 109:2965–71
Angelini A, Melacini P, Barbero F, Thiene G (1999) Evolutionary persistence of spongy myocardium in humans. Circulation 99:2475
Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R (1990) Isolated noncompaction of LV myocardium. A study of eight cases. Circulation 82:507–13
Ichida F, Tsubata S, Bowles KR et al (2001) Novel gene mutations in patients with LV noncompaction or Barth syndrome. Circulation 103:1256–63
Klaassen S, Probst S, Oechslin E et al (2008) Mutations in sarcomere protein genes in LV noncompaction. Circulation 117:2893–901
Sasse-Klaassen S, Gerull B, Oechslin E, Jenni R, Thierfelder L (2003) Isolated noncompaction of the LV myocardium in the adult is an autosomal dominant disorder in the majority of patients. Am J Med Genet A 119A:162–7
Sasse-Klaassen S, Probst S, Gerull B et al (2004) Novel gene locus for autosomal dominant LV noncompaction maps to chromosome 11p15. Circulation 109:2720–3
Martin M, Barriales V, Corros C, Santamaria E (2011) Usefulness of cardiac magnetic resonance imaging in LV non-compaction cardiomyopathy. Eur J Heart Fail 13:177–85
Petersen SE, Selvanayagam JB, Wiesmann F et al (2005) LV non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol 46:101–5
Jacquier A, Thuny F, Jop B et al (2010) Measurement of trabeculated LV mass using cardiac magnetic resonance imaging in the diagnosis of LV non-compaction. Eur Heart J 31:1098–104
Nucifora G, Aquaro GD, Pingitore A, Masci PG, Lombardi M (2011) Myocardial fibrosis in isolated left ventricular non-compaction and its relation to disease severity. Eur J Heart Fail 13:170–6
Elliott P, Anderson B, Arbustini E et al (2008) Classification of the cardiomyopathies: a position statement from the European Society of Cardiology working group on myocardial and pericardial diseases. Eur Heart J 29:270–6
Posch MG, Perrot A, Geier C et al (2009) Genetic deletion of arginine 14 in phospholamban causes dilated cardiomyopathy with attenuated electrocardiographic R amplitudes. Hear Rhythm 6:480–6
Beerbaum P, Barth P, Kropf S et al (2009) Cardiac function by MRI in congenital heart disease: impact of consensus training on interinstitutional variance. J Magn Reson Imaging 30:956–66
Grothoff M, Spors B, Abdul-Khaliq H, Gutberlet M (2008) Evaluation of postoperative pulmonary regurgitation after surgical repair of tetralogy of Fallot: comparison between Doppler echocardiography and MR velocity mapping. Pediatr Radiol 38:186–91
van Geuns RJ, Baks T, Gronenschild EH et al (2006) Automatic quantitative LV analysis of cine MR images by using three-dimensional information for contour detection. Radiology 240:215–21
Udupa JK (1996) Fuzzy connectedness and object definition: theory, algorithms and applications in image segmentation. Graph Model Image Proc 58:246–61
Mosteller RD (1987) Simplified calculation of body-surface area. N Engl J Med 317:1098
Cerqueira MD, Weissman NJ, Dilsizian V et al (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. J Nucl Cardiol 9:240–5
Alfakih K, Plein S, Thiele H et al (2003) Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Res Imaging 17:323–9
Kohli SK, Pantazis AA, Shah JS et al (2008) Diagnosis of left-ventricular non-compaction in patients with left-ventricular systolic dysfunction: time for a reappraisal of diagnostic criteria? Eur Heart J 29:89–95
Weiss F, Habermann CR, Lilje C et al (2003) MRI in the diagnosis of non-compacted ventricular myocardium (NCVM) compared to echocardiography. Rofo 175:1214–9
Probst S, Oechslin E, Schuler P et al (2011) Sarcomere gene mutations in isolated left ventricular noncompaction cardiomyopathy do not predict clinical phenotype. Circ Cardiovasc Genet 4:367–374
Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R (2000) Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol 36:493–500
Junqueira FP, Fernandes FD, Coutinho AC, De Pontes PV, Domingues RC (2009) Case report. Isolated left ventricular myocardium non-compaction: MR imaging findings from three cases. Br J Radiol 82:e37–e41
Marin RC, Ossaba VS, Maroto AE, Sanchez AM (2010) Lack of MR late-enhancement in left ventricular non-compaction in infants and young children. Radiologia 52:138–43
Dodd JD, Holmvang G, Hoffmann U (2007) Quantification of left ventricular noncompaction and trabecular delayed hyperenhancement with cardiac MRI: correlation with clinical severity. Am J Roentgenol 189:974–80
Eitel I, Fürnau G, Walther C et al (2008) Delayed enhancement magnetic resonance imaging in isolated noncompaction of ventricular myocardium. Clin Res Cardiol 97:277–9
Fazio G, Novo G, Casalicchio C et al (2009) Left ventricular non-compaction cardiomyopathy in children: is segmental fibrosis the cause of tissue Doppler alterations and of EF reduction? Int J Cardiol 132:278–80
Huber AM, Schoenberg SO, Hayes C et al (2005) Phase-sensitive inversion-recovery MR imaging in the detection of myocardial infarction. Radiology 237:854–60
Kellman P, Arai AE, McVeigh ER, Aletras AH (2002) Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med 47:372–83
Stoellberger C, Finsterer J (2004) Left ventricular hypertrabeculation/noncompaction. J Am Soc Echocardiography 17:91–100
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Grothoff, M., Pachowsky, M., Hoffmann, J. et al. Value of cardiovascular MR in diagnosing left ventricular non-compaction cardiomyopathy and in discriminating between other cardiomyopathies. Eur Radiol 22, 2699–2709 (2012). https://doi.org/10.1007/s00330-012-2554-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-012-2554-7