Abstract
Purpose
We studied the relationship between doxorubicin pharmacokinetics and body composition in children with cancer.
Patients and methods
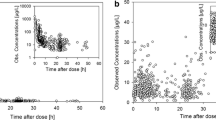
Children between 1 and 21 years of age, receiving doxorubicin as an infusion of any duration <24 h on either a 1-day or 2-day schedule were eligible if they had no significant abnormality of liver function tests, their dose of doxorubicin was not based on ideal body weight or otherwise “capped,” and they weighed ≥12 kg. Body composition was measured by dual-energy X-ray absorptiometry. Doxorubicin and doxorubicinol concentration in plasma were measured by high pressure liquid chromatography. NONMEM was used to perform pharmacokinetic model fitting and S-PLUS was used to perform a post hoc analysis to examine the effect of body composition on pharmacokinetic parameters.
Results
Twenty-two subjects (16 male; 10 Hispanic, 10 Caucasian, 2 Asian) completed the study. The median age was 15.0 years (range 3.3–21.5), median weight was 51.5 kg (range 12.4–80), median BMI was 19.7 (range 13.2–30.0), and median body fat was 25% (range 15–36). The population mean clearance of doxorubicin was 420 ml/min/m2. Doxorubicinol but not doxorubicin clearance was lower in patients with body fat greater than 30%.
Conclusions
Doxorubicinol clearance is decreased in children with >30% body fat. This finding is potentially important clinically, because doxorubicinol may contribute significantly to cardiac toxicity after doxorubicin administration. Further study of the body composition on doxorubicin and doxorubicinol pharmacokinetics and on clinical outcomes is warranted.


Similar content being viewed by others
References
Asayama K, Ozeki T, Sugihara S, Ito K, Okada T, Tamai H, Takaya R, Hanaki K, Murata M (2003) Criteria for medical intervention in obese children: a new definition of ‘obesity disease’ in Japanese children. Pediatr Int 45:642–646
Baker SD, Verweij J, Rowinsky EK, Donehower RC, Schellens JH, Grochow LB, Sparreboom A (2002) Role of body surface area in dosing of investigational anticancer agents in adults, 1991–2001. J Natl Cancer Inst 94:1883–1888
Berg S, Cowan K, Balis F et al (1994) Pharmacokinetics of Taxol and Doxorubicin administered alone and in combination by continuous 72-hour infusion. J Natl Cancer Inst 86:143–145
Bosma RJ, van der Heide JJ, Oosterop EJ, de Jong PE, Navis G (2004) Body mass index is associated with altered renal hemodynamics in non-obese healthy subjects. Kidney Int 65:259–265
Boucek RJ Jr, Olson RD, Brenner DE, Ogunbunmi EM, Inui M, Fleischer S (1987) The major metabolite of doxorubicin is a potent inhibitor of membrane-associated ion pumps. A correlative study of cardiac muscle with isolated membrane fractions. J Biol Chem 262:15851–15856
Callies S, de Alwis DP, Wright JG, Sandler A, Burgess M, Aarons L (2003) A population pharmacokinetic model for doxorubicin and doxorubicinol in the presence of a novel MDR modulator, zosuquidar trihydrochloride (LY335979). Cancer Chemother Pharmacol 51:107–118
Camaggi CM, Comparsi R, Strocchi E, Testoni F, Angelelli B, Pannuti F (1988) Epirubicin and doxorubicin comparative metabolism and pharmacokinetics. A cross-over study. Cancer Chemother Pharmacol 21:221–228
Canal P, Chatelut E, Guichard S (1998) Practical guide for dose individualisation in cancer chemotherapy. Drugs 56:1019–1038
Cheymol G (2000) Effects of obesity on pharmacokinetics. Clin Pharmacokinet 39:215–231
Chinn S (2006) Definitions of childhood obesity: current practice. Eur J Clin Nutr 60:1189–1194
Cindik N, Baskin E, Agras PI, Kinik ST, Turan M, Saatci U (2005) Effect of obesity on inflammatory markers and renal functions. Acta Paediatr 94:1732–1737
Colleoni M, Li S, Gelber RD, Price KN, Coates AS, Castiglione-Gertsch M, Goldhirsch A (2005) Relation between chemotherapy dose, oestrogen receptor expression, and body-mass index. Lancet 366:1108–1110
Crom W, Riley C, Green A et al (1983) Doxorubicin disposition in children and adolescents with cancer. Drug Intell Clin Pharm 17:448
Csernus K, Lanyi E, Erhardt E, Molnar D (2005) Effect of childhood obesity and obesity-related cardiovascular risk factors on glomerular and tubular protein excretion. Eur J Pediatr 164:44–49
Cusack BJ, Young SP, Driskell J, Olson RD (1993) Doxorubicin and doxorubicinol pharmacokinetics and tissue concentrations following bolus injection and continuous infusion of doxorubicin in the rabbit. Cancer Chemother Pharmacol 32:53–58
Dobbs N, James C (1987) Estimation of doxorubicin and doxorubicinol by high performance liquid chromatography and advanced automated sample processor. J Chromatogr Biomed Appl 420:184–188
Doroshow JH (1996) Anthracyclines and anthracenediones. In: Chabner BA, Longo DL (eds) Cancer chemotherapy and biotherapy principles and practice. Lippincott-Raven, Philadelphia, pp 409–434
Eksborg S, Palm C, Bjork O (2000) A comparative pharmacokinetic study of doxorubicin and 4′-epi-doxorubicin in children with acute lymphocytic leukemia using a limited sampling procedure. Anticancer Drugs 11:129–136
Fleming R, Eldridge R, Johnson C, Stewart C (1991) Disposition of high-dose methotrexate in an obese cancer patient. Cancer 68:1247–1250
Forrest GL, Gonzalez B, Tseng W, Li X, Mann J (2000) Human carbonyl reductase overexpression in the heart advances the development of doxorubicin-induced cardiotoxicity in transgenic mice. Cancer Res 60:5158–5164
Frost BM, Eksborg S, Bjork O, Abrahamsson J, Behrendtz M, Castor A, Forestier E, Lonnerholm G (2002) Pharmacokinetics of doxorubicin in children with acute lymphoblastic leukemia: multi-institutional collaborative study. Med Pediatr Oncol 38:329–337
Gelber RP, Kurth T, Kausz AT, Manson JE, Buring JE, Levey AS, Gaziano JM (2005) Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis 46:871–880
Georgiadis MS, Steinberg SM, Hankins LA, Ihde DC, Johnson BE (1995) Obesity and therapy-related toxicity in patients treated for small-cell lung cancer. J Natl Cancer Inst 87:361–366
Gibbs JP, Gooley T, Corneau B, Murray G, Stewart P, Appelbaum FR, Slattery JT (1999) The impact of obesity and disease on busulfan oral clearance in adults. Blood 93:4436–4440
Green B, Duffull SB (2004) What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol 58:119–133
Griggs JJ, Sorbero ME, Lyman GH (2005) Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med 165:1267–1273
Gurney H (2002) How to calculate the dose of chemotherapy. Br J Cancer 86:1297–1302
Hempel G, Flege S, Wurthwein G, Boos J (2002) Peak plasma concentrations of doxorubicin in children with acute lymphoblastic leukemia or non-Hodgkin lymphoma. Cancer Chemother Pharmacol 49:133–141
Hijiya N, Panetta JC, Zhou Y, Kyzer EP, Howard SC, Jeha S, Razzouk BI, Ribeiro RC, Rubnitz JE, Hudson MM, Sandlund JT, Pui CH, Relling MV (2006) Body mass index does not influence pharmacokinetics or outcome of treatment in children with acute lymphoblastic leukemia. Blood 108:3997–4002
Joerger M, Huitema AD, Meenhorst PL, Schellens JH, Beijnen JH (2005) Pharmacokinetics of low-dose doxorubicin and metabolites in patients with AIDS-related Kaposi sarcoma. Cancer Chemother Pharmacol 55:488–496
Joerger M, Huitema AD, Richel DJ, Dittrich C, Pavlidis N, Briasoulis E, Vermorken JB, Strocchi E, Martoni A, Sorio R, Sleeboom HP, Izquierdo MA, Jodrell DI, Fety R, de Bruijn E, Hempel G, Karlsson M, Tranchand B, Schrijvers AH, Twelves C, Beijnen JH, Schellens JH (2007) Population pharmacokinetics and pharmacodynamics of doxorubicin and cyclophosphamide in breast cancer patients: a study by the EORTC-PAMM-NDDG. Clin Pharmacokinet 46:1051–1068
Lange BJ, Gerbing RB, Feusner J, Skolnik J, Sacks N, Smith FO, Alonzo TA (2005) Mortality in overweight and underweight children with acute myeloid leukemia. JAMA 293:203–211
Lee K, Lee S, Kim SY, Kim SJ, Kim YJ (2007) Percent body fat cutoff values for classifying overweight and obesity recommended by the International Obesity Task Force (IOTF) in Korean children. Asia Pac J Clin Nutr 16:649–655
Liew PL, Lee WJ, Lee YC, Wang HH, Wang W, Lin YC (2006) Hepatic histopathology of morbid obesity: concurrence of other forms of chronic liver disease. Obes Surg 16:1584–1593
Lind MJ, Margison JM, Cerny T, Thatcher N, Wilkinson PM (1989) Prolongation of ifosfamide elimination half-life in obese patients due to altered drug distribution. Cancer Chemother Pharmacol 25:139–142
Mushlin PS, Cusack BJ, Boucek RJ Jr, Andrejuk T, Li X, Olson RD (1993) Time-related increases in cardiac concentrations of doxorubicinol could interact with doxorubicin to depress myocardial contractile function. Br J Pharmacol 110:975–982
Nanda K (2004) Non-alcoholic steatohepatitis in children. Pediatr Transplant 8:613–618
Olson LE, Bedja D, Alvey SJ, Cardounel AJ, Gabrielson KL, Reeves RH (2003) Protection from doxorubicin-induced cardiac toxicity in mice with a null allele of carbonyl reductase 1. Cancer Res 63:6602–6606
Olson RD, Mushlin PS, Brenner DE, Fleischer S, Cusack BJ, Chang BK, Boucek RJ Jr (1988) Doxorubicin cardiotoxicity may be caused by its metabolite, doxorubicinol. Proc Natl Acad Sci U S A 85:3585–3589
Palle J, Frost BM, Peterson C, Gustafsson G, Hellebostad M, Kanerva J, Schmiegelow K, Lonnerholm G (2006) Doxorubicin pharmacokinetics is correlated to the effect of induction therapy in children with acute myeloid leukemia. Anticancer Drugs 17:385–392
Poikonen P, Blomqvist C, Joensuu H (2001) Effect of obesity on the leukocyte nadir in women treated with adjuvant cyclophosphamide, methotrexate, and fluorouracil dosed according to body surface area. Acta Oncol 40:67–71
Ribbing J, Jonsson EN (2004) Power, selection bias and predictive performance of the Population Pharmacokinetic Covariate Model. J Pharmacokinet Pharmacodyn 31:109–134
Rodvold KA, Rushing DA, Tewksbury DA (1988) Doxorubicin clearance in the obese. J Clin Oncol 6:1321–1327
Rosner GL, Hargis JB, Hollis DR, Budman DR, Weiss RB, Henderson IC, Schilsky RL (1996) Relationship between toxicity and obesity in women receiving adjuvant chemotherapy for breast cancer: results from cancer and leukemia group B study 8541. J Clin Oncol 14:3000–3008
Santos CA, Boullata JI (2005) An approach to evaluating drug-nutrient interactions. Pharmacotherapy 25:1789–1800
Stewart DJ, Grewaal D, Green RM, Mikhael N, Goel R, Montpetit VA, Redmond MD (1993) Concentrations of doxorubicin and its metabolites in human autopsy heart and other tissues. Anticancer Res 13:1945–1952
Wang GX, Wang YX, Zhou XB, Korth M (2001) Effects of doxorubicinol on excitation–contraction coupling in guinea pig ventricular myocytes. Eur J Pharmacol 423:99–107
Acknowledgments
This work was funded in part by the Thrasher Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thompson, P.A., Rosner, G.L., Matthay, K.K. et al. Impact of body composition on pharmacokinetics of doxorubicin in children: a Glaser Pediatric Research Network study. Cancer Chemother Pharmacol 64, 243–251 (2009). https://doi.org/10.1007/s00280-008-0854-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-008-0854-z