Abstract
Background
Gastrointestinal motility disturbances in critically ill patients are frequent in the ICU setting, causing considerable discomfort and are associated with increased rates of morbidity and mortality. This review focuses on the pathophysiological basis of intestinal motility, the major patterns of pathological motility alterations, the impact on patient outcome, and current therapeutic options.
Discussion
Intestinal motility is controlled by the enteric nervous system, modulated by hormones and extrinsic afferent and efferent neurons. Pathological motility disturbances can affect the stomach, small bowel, and colon separately or in combination. Changes in esophageal motor activity contribute to the aspiration of gastric juice, whereas early enteral feeding most frequently fails due to gastric intolerance. Disturbances in digestive and interdigestive motility patterns and the inability to switch motor activity from the interdigestive to the digestive pattern also contribute to feeding disability and thus to increased morbidity and mortality as well.
Conclusions
The therapeutic options for motility disturbances in critically ill patients include the adjustment of electrolyte imbalances, tailored fluid management, early enteral feeding, appropriate management of catecholamines and drugs used for analgosedation, and prokinetic drugs. Unfortunately, the therapeutic options for treating motility disturbances in ICU patients are still limited. This situation requires careful assessment of ICU patients with respect to gut motility disturbances and their pathophysiological mechanisms and an individually tailored treatment to prevent further aggravation of existing motility disturbances.
Similar content being viewed by others
Introduction
In our intensive care units (ICU) we observe two scenarios when patients develop gastrointestinal (GI) motility disturbances: patients who develop GI paralysis in the immediate postoperative period and critically ill patients who suffer from GI motility disorders. The occurrence of GI motility disturbances in critically ill patients is well known. Abnormalities in gastric emptying affect 50% of mechanically ventilated patients and 80% of patients with increased cranial pressure after head injury [1, 2]. These GI motility disturbances cause considerable discomfort to the patient (nausea, vomiting, flatulence), and they are also associated with an increased rate of complications-ventilator-associated pneumonia, infections, the risk of bacterial translocation, and the inability to be fed [3, 4]. Unfortunately, there are outcome data related to GI motility disturbances during the postoperative period but not for critically ill patients. When the postoperative period is complicated by motility disturbances, significantly higher rates of deep venous thromboses and pulmonary artery embolisms are seen in patients undergoing hip or knee arthroplasty [5]. Postoperative ileus, per se or aggravated by other complications, is also a common cause of prolonged hospitalization and a significant factor contributing to hospital readmission [6, 7]. During the postoperative period increased morbidity and mortality rates, prolonged hospital stay, and higher rates of hospital readmission due to postoperative ileus increase health care costs [8].
The topic of GI motility derangements in the ICU setting is of substantial relevance to intensivists. After briefly describing the pattern of normal digestive and interdigestive small bowel motility, this review addresses the pathophysiological basis of motility disturbances. Esophageal and gastric motility and their disturbances are also briefly touched upon. A major part of the article focuses on the beneficial and adverse effects of ICU treatments on gut motility.
Normal gastrointestinal motility
To maintain fluid, electrolyte, and energy homeostasis by the assimilation of food the gut has not only the most extensive immune system in the body but also the largest collection of neurons (up to 108 cells) outside the central nervous system [9, 10]. This enteric nervous system (ENS) is located within the bowel wall, extending from the esophagus to the internal anal sphincter, and is organized in such a way that it can operate independently of the brain [9]. The neurons of the ENS are arranged in two main plexuses (Fig. 1). The myenteric plexus located between the longitudinal and circular muscle layers mainly regulates motility while the submucous plexus between the circular muscle and the mucosa is involved in the regulation of mucosal processes (electrolyte and fluid secretion, mucus secretion, mucosal blood flow and neuroimmune interactions) [11]. The ENS in the guinea pig intestine contains more than 15 types of neurons [12]. The major classes of neurons are intrinsic primary afferent neurons, interneurons connecting the ganglia within the gut and between the plexuses, motor neurons, secretomotor neurons, and vasodilator neurons [12]. While the intrinsic primary afferent neurons supply the ENS with information for the appropriate control of digestion, the extrinsic afferents provide the brain with relevant data for fluid and energy homeostasis, mediate the sensation of pain and discomfort, and regulate immune, inflammatory, and pathological processes [9]. The primary transmitters in the excitatory motor neurons are acetylcholine and substance P, while the transmitters in the inhibitory neurons are nitric oxide, vasoactive intestinal peptide, and adenosine triphosphate. A loss of inhibitory neurons would lead to hypermotility, while hyperactivity of inhibitory neurons or impaired function of excitatory neurons would cause a loss of tone in the gut, both resulting in severe intestinal motility disturbances [11].
Schematic diagram showing the innervation of the gastrointestinal tract by intrinsic enteric neurons originating in the myenteric plexus and submucosal plexus, extrinsic sensory neurons originating from the nodose ganglia and dorsal root ganglia, and autonomic efferent neurons of the parasympathetic nervous system (vagus and pelvic nerves) and sympathetic nervous system (splanchnic nerves). (Modified with permission from Holzer et al. 2001 [9])
Esophageal motility
The musculature of the esophagus is made up of skeletal muscle in the upper third, a mixture of skeletal and smooth muscle in the middle, and smooth muscle only in the lower third. Its assigned task is to transport the swallowed food into the stomach. After swallowing the food (a voluntary act) the two (upper and lower) esophageal sphincters relax and open while a peristaltic contraction propels the food bolus [13]. After the contraction has swept through the entire length of the esophagus, the sphincters close. Peristalsis in the upper skeletal muscle part of the esophagus is the result of the sequential activation of neurons in the nucleus ambiguus, one of the vagal motor nuclei. Peristalsis in the smooth muscle part of the esophagus is mediated at the level of the dorsomotor nucleus of the vagal nerve and at the level of the myenteric plexus [13].
Gastrointestinal motility
Recordings of small bowel motility, comparable to the motility found in the antrum of the stomach, can be subdivided into interdigestive (fasting) and digestive (feeding) motility patterns [14].
Gastric emptying
The fundus, the upper part of the stomach, is involved predominantly in the accommodation of food. The underlying relaxation of the proximal gastric wall seems to be mediated by inhibitory vagal neurons because this reflex is abolished in vagotomy patients [15]. Digestible substances are emptied from the stomach when they have reached an essentially liquid form [15]. The distal part of the stomach, the antrum, seems responsible for reducing solids to the required fluid form. This part of the stomach is also controlled by neuronal and humoral mechanisms. The composition of the gastric contents influences the rate of gastric emptying. Neutral, isoosmolar, and calorically inert fluids empty fast while hypertonic fluids containing acid, fat or certain amino acids delay gastric emptying [15].
Interdigestive motility pattern
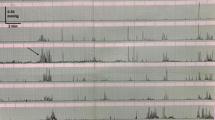
The interdigestive motility pattern, the migrating motor complex (MMC), starts several hours after a meal has passed through the stomach and small intestine, progresses to the distal ileum, and consists of three distinct phases that are repeated approx. every 2 h: phase I is a period of quiescence (45–60 min), phase II a period of irregular contractions with a duration of 30–45 min, and phase III a period of regular, propulsive activity that lasts 5–15 min (Fig. 2) [15]. However, it should be noted that the duration of these MMC periods shows enormous inter- and intraindividual variability [16]. Previously only the phase III was referred to as the MMC, but now all three phases of interdigestive motility are subsumed under this term.
Pattern of a normal nocturnal migrating motor complex (MMC) recorded in the duodenum (upper tracing) and proximal jejunum (lower tracing) of a healthy elderly woman. Lower panel shows a short period in high resolution. Phase III is preceded by phase II with some contractile activity, usually limited during sleep, and succeeded by phase I. (Modified with permission from Husebye et al. [76])
By the time one MMC ends in the ileum, a new one begins in the stomach. A normal MMC pattern is important for the purging of the small bowel. During phase III remaining food particles and indigestible residues are swept to the distal part of the small bowel and into the colon. An undisturbed MMC may also play a role in protection against bacterial overgrowth of remaining food particles in the small bowel [14]. The MMC is controlled by the ENS and modulated by regulatory peptides, while a disconnection of the extrinsic nerve supply does not interrupt the MMC pattern [17]. One of the MMC-regulating peptides is motilin. Intravenous administration of motilin or the motilin receptor agonist erythromycin initiates phase III activity in the antroduodenal region, while somatostatin and other substances can initiate phase III activity in the duodenum [16]. An absence of the MMC indicates severe enteric dysfunction, while the presence of the MMC is thought to predict a successful outcome of enteral feeding [14, 16, 18].
Digestive motility pattern
Ingestion of a meal disrupts the interdigestive motility pattern and replaces it with accommodation, stationary motility (segmental contractions and pendular movements), and propulsive peristalsis [9]. Accommodation refers to an active relaxation of the fundus in response to food intake. Accommodation is also present in the small bowel and colon where filling of the bowel initiates a descending inhibitory reflex pathway [9, 19]. Stationary contractions mix the food with the secretions of the gut and improve its contact with the mucosa of the small intestine to allow absorption of luminal contents. These stationary contractions are due to pendular movements of the longitudinal muscle and segmental contractions of the circular muscle [9]. Their rhythm comes from the interstitial cells of Cajal [20]. These cells are important transducers of the ENS output to the musculature as they are innervated by excitatory and inhibitory motor neurons [20]. Their oscillating membrane potential, transmitted electrically to the adjacent smooth muscle layers, initiates slow waves with a frequency of 10/min, and these slow waves determine the frequency of the muscular contractions. Propulsive peristalsis is initiated by distortion of the mucosal villi or distension of the gut wall and involves a contraction of the circular muscle orally and relaxation aborally of the site of the stimulus [9]. The propulsive peristaltic wave progresses only a few centimeters in order to forward the chyme, but not as far as phase III of the MMC in the interdigestive motility pattern. The digestive pattern persists for several hours after ingestion of a meal. The duration seems to depend mainly on the caloric load of the food: the higher the caloric load, the longer the duration is [16, 21].
Patterns of motility disturbances
Pathological motility disturbances differ in clinical appearance and location. They can affect the stomach, small bowel, and colon separately or in combination. GI motility disturbances are a common complication in patients suffering from severe hemodynamic instability, multiple organ failure, intra-abdominal hypertension or intra-abdominal compartment syndrome. The opposite can also be the case, with motility disturbances per se causing the above comorbidities. Abdominal compartment syndrome, for example, is associated with intestinal ischemia and leads to multiple organ failure and ileus. On the other hand, ileus and intestinal dilatation with increased luminal pressures cause gut wall ischemia, intra-abdominal hypertension and intra-abdominal compartment syndrome. Regardless of what initiates the vicious circle, motility disturbances in these patients are associated with higher morbidity and mortality rates.
Disturbances in esophageal motility
Esophageal motility disturbances are frequent in patients suffering from diabetes, alcoholism, or other systemic diseases [13]. In critically ill patients the frequency and amplitude as well as the percentage of propulsive contractions of the esophagus are reduced [22]. Several drugs used in critically ill patients—ketamine, benzodiazepines, and opioids—seem to inhibit esophageal motor activity [22]. Changes in esophageal motor activity and a decrease in the pressure of the lower gastroesophageal sphincter result in regurgitation of gastric contents and consequently in aspiration of gastric juice [22].
Disturbances in gastric emptying
Gastric intolerance is the most frequent reason why attempts to institute early enteral feeding fail [3, 4, 23]. Impaired gastric emptying may lead to a discrepancy between the delivered and prescribed volume of feed and an increased risk of pulmonary aspiration and bacterial overgrowth [23, 24]. One mechanism that impairs gastric emptying is a primary motor dysfunction resulting in antral hypomotility and the presence of the fasting motility pattern during feeding [25]. Another mechanism that causes inhibition of gastric emptying involves an inhibitory feedback pathway originating in the proximal small bowel [24]. Nutrients release neuroendocrine peptides such as cholecystokinin (CCK, acting peripherally via CCK1 receptors) and serotonin [5-hydroxytryptamine (5-HT), acting via 5-HT3 receptors) that activate vagal and spinal afferent neurons and thus inhibit gastric emptying [26].
Several other factors such as hyperglycemia, increased intracranial pressure, and stress are known to affect gastric emptying [2, 27]. Hyperglycemia, for example, seems to inhibit gastric emptying by reducing vagal efferent activity and inhibition of the release of nitric oxide from the myenteric plexus [27]. During periods of hyperglycemia the effect of prokinetic drugs such as erythromycin and cisapride on gastric emptying may be adversely affected [27, 28].
Disturbances in the interdigestive motility pattern
Disturbances of the MMC are frequent in critically ill patients. Miedema and coworkers [29] reported that postoperative MMCs differ from those in control subjects in having more phase I, less phase II, and more frequent phase III activity. Importantly, peristaltic contractions in phase III may tend to be retrograde rather than antegrade and thus significantly delay small bowel transit [29]. These findings compare well with other published studies. In mechanically ventilated patients MMC activity fronts are totally absent in the antrum but originate from the duodenum. The length of the MMC is comparable to that in healthy volunteers, but there is an increase in phase I activity and a decrease in phase II activity [30]. In addition, the propagation of the MMC is abnormal, with retrograde or stationary activity fronts in several patients [30]. MMC disturbances reduce flushing of the luminal contents (bacteria, food remnants and cell detritus) into the colon, which results in stagnation, microbial overgrowth, bacterial translocation and a vicious circle that might lead to increased occurrence of ventilator-associated pneumonia [31, 32]. These findings were confirmed by Bosscha and coworkers [33] who reported that in more than 90% of mechanically ventilated patients with morphine administration phase III of the MMC started in the duodenum and not in the antrum. This disturbed the MMC-associated purging of the stomach, resulted in antral hypomotility and was related to the severity of gastric retention [33].
Disturbances in the digestive motility pattern
A frequent problem in critically ill patients is the inability to switch motor activity from the interdigestive to the digestive pattern. These patients develop pressure waves, comparable to phase III activity of the MMC except that they occur despite nutrient delivery to the gut. The frequency of these pressure waves is at least double that of normal interdigestive motor activity [25, 33]. The failure of the motor activity to convert from the interdigestive to the digestive pattern may be a factor contributing to the high occurrence of diarrhea in critically ill patients [3].
Acute colonic pseudo-obstruction (Ogilvie's syndrome)
In the colon of critically ill patients a variant of motility disturbances is the acute colonic pseudo-obstruction (Ogilvie's syndrome). Clinical symptoms are abdominal discomfort and distension. Passage of stool or gas is frequently but not always absent; up to 41% of patients still pass gas or even experience diarrhea [34]. Abdominal films show a diffuse dilatation of the colon with normal mucosal markings and haustra, and a thin colonic wall (in contrast to toxic colitis), while small bowel dilatation is mostly absent [35]. Acute colonic pseudo-obstruction is frequent in diseases such as gut ischemia or systemic or local inflammation (abdomen, urinary tract or thorax). Mortality rates are high but depend on the underlying condition and the speed of therapeutic interventions (15% in patients with early decompression and 44% in patients with perforated or ischemic bowel) [36]. The risk of perforation is approx. 3%; risk factors for perforation include cecal diameter, increased age, and duration of distension [35].
Drugs with possible adverse side effects on motility
Many of the drugs used in the ICU, including analgesic drugs or vasoactive substances for hemodynamic stabilization, contribute significantly to the origin and persistence of motility disturbances in critically ill patients.
Opioids
Endogenous opioids modulate a variety of biological processes including stress response, immunity, analgesia, motor activity, and autonomic function. In the GI tract opioid peptides have been localized to enteric neurons and endocrine cells [37]. Opiate drugs activate the same receptors as native opioids [37]. These inhibit gastrointestinal transit by inhibiting neurotransmitter release and by changing neuronal excitability. In addition to the effects on motility, electrolyte and fluid transport is modulated via stimulation of water and electrolyte absorption as well as modulation of gastric acid and bicarbonate secretion [38]. The undesirable inhibitory side effects of opioids on intestinal motility have long been known, but the extent of their inhibitory potential on intestinal motility may be underestimated [39]. An animal model demonstrated that one-quarter of the dose needed to produce analgesia inhibits intestinal motility and one-twentieth of the analgesic dose is sufficient to stop diarrhea [39]. In contrast to many other opioid-induced side effects such as nausea, vomiting and sedation, patients rarely develop tolerance to the constipating effects of opioids [40].
Paracetamol
Paracetamol, whose analgesic effect is possibly weaker (20–30%) than that of nonsteroidal anti-inflammatory drugs and selective cyclooxygenase-2 inhibitors, has been thought to have almost no intestinal side effects at recommended doses [41]. In a well established experimental setting, however, paracetamol was recently demonstrated to have an inhibitory effect on guinea pig peristalsis while acetylsalicylic acid and metamizole did not [42].
α2-Adrenoceptor agonists
The α2-adrenoceptor agonists clonidine and dexmedetomidine (currently available in the context of clinical investigations) have gained in importance as additive analgesic drugs and as effective therapeutic options for patients with alcohol withdrawal syndromes [43]. They induce sedation, reduce anesthetic and analgesic requirements, and improve perioperative hemodynamic and sympathoadrenal stability [44]. On the other hand, they inhibit gastric, small bowel, and colonic motility in animal and human studies [45, 46, 47].
Catecholamines
Catecholamines are often essential to achieve and maintain hemodynamic stability in ICU patients. In vitro data demonstrate a direct, dose-dependent inhibitory effect of all clinically used catecholamines on small bowel motility [48]. In vivo data are available only for dopamine, demonstrating a direct inhibitory effect on GI motility during fasting and nasogastric feeding [49]. Moreover, most patients treated with dopamine fail to convert their fasting motility to the feeding pattern after the onset of enteral nutrition [49].
Fluid and electrolyte management
Inadequate fluid management and salt overload may result in peripheral, pulmonary, and splanchnic edema, potentially aggravating preexisting motility disturbances and impairing enteral nutrition [50]. Unfortunately, the available data pertain only to fluid management in the perioperative period and not selectively in the ICU. In general, the studies demonstrate that liberal fluid management (12 vs. 4 ml/kg per hour) prolongs the duration of motility disturbances and is associated with longer latencies to first gastric emptying and first passage of flatus and stool as well as to hospital discharge, compared with restrictive management [51, 52]. A direct effect of electrolyte abnormalities on intestinal motility has been demonstrated only for potassium and magnesium. Increased potassium and magnesium values reduce the duration of ileus [53, 54].
Starvation
Several studies report the effect of starvation on gut function. In experimental animals a long period of starvation is associated with mucosal atrophy and reduced enzymatic activity [55]. In humans there is evidence of a delay in gastric emptying, which is correlated with the degree of malnutrition and severe atrophy of the stomach and small bowel wall and carries a risk of distension [56]. Patients with malnutrition suffer from constipation and a longer orocecal transit time that tend to normalize with normal eating and the return to a suitable body weight [56].
Effective methods to treat intestinal motility disturbances
Therapeutic options for motility disturbances in critically ill patients include the adjustment of electrolyte imbalances, tailored fluid management (2–3 l/day), early enteral feeding, and appropriate management of catecholamines and drugs used for analgosedation. Stool softeners are well tolerated but are ineffective if fluid intake is inadequate, and they usually are ineffective when administered alone [57].
Stomach and small bowel
Metoclopramide is a dopamine D2 receptor antagonist with central and peripheral effects as well as being a 5-HT3 receptor antagonist and a 5-HT4 receptor agonist (Table 1). Metoclopramide increases gastric motility and has a moderate prokinetic effect on the small bowel [58]. The substance may be useful in critically ill patients but seems to be ineffective in patients with postoperative ileus [59]. For patients with renal failure, the recommended intravenous dose of 10 mg three times daily must be reduced gradually; the recommended dose for hemodialysis patients is 10 mg/day [60].
Domperidone is a drug that acts primarily as an antagonist at peripheral dopamine D2 receptors. In contrast to metoclopramide, the substance does not cross the blood-brain barrier and is therefore free of central nervous side effects. The recommended dose is 10 mg orally three times daily.
Cisapride and tegaserod are both 5-HT4 receptor agonists. Cisapride, which because of Q-T interval prolongation and ventricular arrhythmias is no longer available, enhances esophageal peristalsis and increases lower esophageal tone and gastric emptying. Tegaserod accelerates gastric emptying and shortens small bowel and colonic transit time [61]. Studies evaluating the effect of tegaserod on motility disturbances in critically ill patients are still scarce, and currently there is only one report demonstrating a positive effect of tegaserod on gastric emptying and vomiting [62].
The macrolide erythromycin stimulates GI motility by acting on motilin receptors on smooth muscle cells and enteric neurons to facilitate neurotransmitter release [63]. Erythromycin effectively improves gastric emptying, and there is a trend towards shorter times necessary for tube placement [58]. The recommended dose is 250 mg orally twice daily or 1–3 mg/kg intravenously every 6 h [60]. Newer studies evaluating the duration of erythromycin's action indicate that a dose given twice daily may be sufficient for critically ill patients who do not tolerate enteral feeding [64]. Long-term use, however, seems to be limited by erythromycin's antibacterial action and desensitization to the therapeutic effects of the drug [63]. The recommended dose thus should not be given for more than 3 or 4 days.
Octreotide, a synthetic peptide, has a sequence of four essential amino acids in common with somatostatin but a longer duration of action than somatostatin; it acts via induction of the MMC. The effect of octreotide on small bowel motility is controversial and seems to depend on the dose range tested, because higher doses (> 150 mg/day) may inhibit motility [60].
Small bowel and colon
Cerulein, a potent CCK-like decapeptide, activates CCK receptors on enteric neurons, and stimulates small bowel motility by releasing excitatory transmitters such as acetylcholine and substance P (Table 1). The pronounced prokinetic effect of clinically used doses of cerulein (0.15–0.3 μg/kg per day) has been confirmed in an experimental setting [65]. An occasional side effect of cerulein is prolongation of gastric emptying, an effect that may be prevented by metoclopramide or erythromycin.
Neostigmine, an inhibitor of acetylcholine esterase and thus an indirect cholinergic agonist, has been reported to reduce the time to first passage of gas and stool. Interestingly, the lower the administered dose (2–2.5 vs. 9.6 mg/24 h), the shorter is the time to the passage of gas and stool [66, 67, 68]. These findings are explained by experimental data demonstrating a moderate prokinetic effect that is limited to a narrow concentration window, while higher doses inhibit small bowel motility [65] (Fig. 3).
Ogilvie's syndrome: therapeutic options
Colonic diameters should be monitored daily with abdominal radiographs. When the diameter of the intestine is greater than 9 cm, colonoscopic decompression should be performed within 72 h after the diagnosis to reduce the risk of death [36, 69]. Nasogastric tubes can help to decrease the amount of swallowed air and relieve vomiting. A rectal tube is helpful when colonic distension extends to the rectosigmoid region [35]. Neostigmine and tegaserod may be promising drugs for these patients [66].
Future therapeutic considerations
Currently several substances are under evaluation. Ghrelin, discovered in the endocrine cells of the stomach, is not only an appetite-related peptide with highest levels in the fasting state, a sharp rise before a meal, and a fall within 1 hour after a meal. It also accelerates gastric emptying and small bowel transit in animal models [70, 71]. Another promising drug may be fedotozine, a κ-opioid receptor agonist with good analgesic effects and improved bowel function in animal studies [72].
In recent years the use of opioid antagonists to reduce the intestinal side effects of opioid analgesics has been increasingly discussed. The quaternary opioid antagonists alvimopan (ADL 8-2698) and methylnaltrexone (MNTX) antagonize the inhibitory effect of opioids on gut motility but do not cross the blood-brain barrier and therefore do not antagonize the analgesic effect of opiates. MNTX proved effective in phase 2 studies, while alvimopan reduced nausea and vomiting in postoperative patients and restored GI function earlier than in control patients [73, 74].
Lipids and fatty acids induce the release of CCK from duodenal endocrine cells. CCK inhibits gastric motility and gastric emptying. Cholecystokinin receptor antagonists such as loxiglumide and dexloxiglumide block the inhibitory effect of a lipid meal on gastric motility and gastric emptying [63]. CCK receptor antagonists also reduce the occurrence of meal-like fullness and nausea and increase the intraluminal pressures at which sensations are reported [75].
In conclusion, many of the drugs currently used in the ICU, particularly catecholamines and opioid analgesics, exert inhibitory effects on intestinal peristalsis. The therapeutic options for treating motility disturbances in ICU patients are still limited. This situation requires careful assessment of ICU patients as well as interpretation of the underlying pathophysiological mechanisms, and an individual therapeutic approach to prevent further aggravation of existing motility disturbances to improve patients' outcomes and to decrease health care costs.
References
Tarling MM, Toner CC, Withington PS, Baxter MK, Whelpton R, Goldhill DR (1997) A model of gastric emptying using paracetamol absorption in intensive care patients. Intensive Care Med 23:256–260
Kao CH, ChangLai SP, Chieng PU, Yen TC (1998) Gastric emptying in head-injury patients. Am J Gastroenterol 93:1108–1112
Ritz MA, Fraser R, Tam W, Dent J (2000) Impacts and patterns of disturbed gastrointestinal function in critically ill patients. Am J Gastroenterol 95:3044–3052
Herbert MK (1999) Impairment of intestinal motility in the critically ill patient. Clinical implications and contribution of drugs and mediators. In: Herbert MK, Holzer P, Roewer N (ed) Problems of the gastrointestinal tract in anesthesia, the perioperative period and intensive care. Springer, Berlin Heidelberg New York, pp 28–38
Berend KR, Lombardi AV Jr, Mallory TH, Dodds KL, Adams JB (2004) Ileus following total hip or knee arthroplasty is associated with increased risk of deep venous thrombosis and pulmonary embolism. J Arthroplasty 19:82–86
Goodney PP, Stukel TA, Lucas FL, Finlayson EV, Birkmeyer JD (2003) Hospital volume, length of stay, and readmission rates in high-risk surgery. Ann Surg 238:161–167
Luckey A. Livingston E, Tache Y (2003) Mechanisms and treatment of postoperative ileus. Arch Surg 138:206–214
Prasad M, Matthews JB (1999) Deflating postoperative ileus. Gastroenterology 117:489–492
Holzer P, Schicho R, Holzer-Petsche U, Lippe I (2001) The gut as a neurological organ. Wien Klin Wochenschr 18:647–660
Furness JB, Kunze WAA, Bertrand PP, Clerc N, Bornstein JC (1998) Intrinsic primary afferent neurons of the intestine. Prog Neurobiol 54:1–18
Schemann M, Reiche D, Neunlist M (1999) Properties and functional aspects of the enteric nervous system In: Herbert MK, Holzer P, Roewer N (ed) Problems of the gastrointestinal tract in anesthesia, the perioperative period and intensive care. Springer, Berlin Heidelberg New York, pp 3–11
Costa M, Brookes SJH, Steele PA, Gibbins I, Burcher E, Kandiah CJ (1996) Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience 75:949–967
Mittel RK, Bhalla V (2004) Oesophageal motor functions and its disorders. Gut 53:1536–1542
Vantrappen G, Janssens J, Hellemans J, Ghoos Y (1977) The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J Clin Invest 59:1158–1166
Minami H, McCallum RW (1984) the physiology and pathophysiology of gastric emptying in humans. Gastroenterology 86:1592–1610
Husebye E (1999) The patterns of small bowel motility: physiology and implications in organ disease and functional disorders. Neurogastroenterol Motil 11:141–161
Sarna SK (1985) Cyclic motor activity, migrating motor complex: 1985. Gastroenterology 89:894–913
Schippers E, Holscher AH, Bollschweiler E, Siewert JR (1991) Return of interdigestive motor complex after abdominal surgery: end of postoperative ileus? Dig Dis Sci 36:621–626
Costa M, Brooks SJ, Hennig GW (2000) Anatomy and physiology of the enteric nervous system. Gut 47 [Suppl]:15–19
Huizinga JD, Robinson TL, Thomsen L (2000) The search of the origin of rhythmicity in intestinal contraction: from tissue to single cells. Neurogastroenterol Motil 12:3–9
Ouyang A, Sunshine AG, Reynolds JC (1989) Caloric content of a meal affects duration but not contractile pattern of duodenal motility in man. Dig Dis Sci 34:528–536
Kölbel C, Rippel K, Klar H, Singer MV, van Ackern K, Fiedler F (2000) Esophageal motility disorders in critically ill patients: a 24-hour manometric study. Intensive Care Med 26:1421–1427
Adam S, Batson S (1997) A study of problems with delivery of enteral feed in critical ill patients in five ICUs in the UK. Intensive Care Med 23:261–266
Chapman M, Fraser R, Vozzo R, Bryant L, Tam W, Nguyen N, Zacharakis B, Butler R, Davidson G, Horowitz M (2005) Antro-pyloro-duodenal motor responses to gastric and duodenal nutrient in critically ill patients. Gut 54:1384–1390
Dive A, Miesse C, Jamart J (1994) Duodenal motor response to continuous enteral feeding is impaired in mechanically ventilated patients. Clin Nutr 13:302
Raybould HE, Glatzle J, Robin C, Meyer JH, Phan T, Wong H, Sternini C (2003) Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. Am J Physiol Gastrointest Liver Physiol 284:G367–G372
Spallone V (2004) Glycaemic control and gastric emptying. Diabetes Nutr Metab 17:47–55
Deehan S, Dobb GJ (2002) Metoclopramide-induced raised intracranial pressure after head injury. J Neurosurg Anesthesiol 14:157–160
Miedema BW, Schillie S, Simmons JW, Burgess SV, Liem T, Silver D (2002) Small bowel motility and transit after aortic surgery. J Vasc Surg 36:19–24
Dive A, Moulart M, Jonard P, Jamart J, Mahieu P (1994) Gastroduodenal motility in mechanically ventilated critically ill patients: a manometric study. Crit Care Med 22:441–447
Gunnarsdottir SA, Sadik R, Shev S, Simren M, Sjovall H, Stotzer PO, Abrahamsson H, Olsson R, Bjornsson ES (2003) Small intestinal motility disturbances and bacterial overgrowth in patients with liver cirrhosis and portal hypertension. Am J Gastroenterol 98:1362–1370
Torres A, el-Ebiary M, Soler N, Monton C, Gonzalez J, Puig de la Bellacasa J (1995) The role of the gastric reservoir in ventilator-associated pneumonia. Clin Intensive Care 6:174–180
Bosscha K, Nieuwenhuijs VB, Vos A, Samsom M, Roelofs JM, Akkermans LM (1998) Gastrointestinal motility and gastric tube feeding in mechanically ventilated patients. Crit Care Med 26:1510–1517
Delgado Aros S, Camilleri M (2003) Pseudo-obstruction in the critically ill. Best Pract Res Clin Gastroenterol 17:427–444
Fazel A, Verne GN (2005) New solutions to an old problem: acute colonic pseudo-obstruction. J Clin Gastroenterol 39:17–20
Vanek VW, Al Salti M (1986) Acute pseudo-obstruction of the colon (Ogilvie's syndrome). An analysis of 400 cases. Dis Colon Rectum 29:203–210
Sternini C, Patierno S, Selmer IS, Kirchgessner A (2004) The opioid system in the gastrointestinal tract. Neurogastroenterol Motil 16:3–16
Kromer W (1988) Endogenous and exogenous opioids in the control of gastrointestinal motility and secretion. Pharmacol Rev 40:121–162
Yuan C, Foss J (2000) Methylnaltrexone: Investigation of clinical applications. Drug Dev Res 50:133–141
Walsh TD (1990) Prevention of opioid side effects. J Pain Symptom Manage 5:362–7
Kehlet H, Dahl JB (2003) Anaesthesia, surgery, and challenges in postoperative recovery. Lancet 362:1921–1928
Herbert MK, Weis R, Holzer P, Roewer N (2005) Peristalsis in the Guinea pig small intestine in vitro is impaired by acetaminophen but not aspirin and dipyrone. Anesth Analg 100:120–127
Jones MEP, Maze M (2001) Editorial I-Can we characterize the central nervous system actions of α2-adrenergic agonists? Br J Anaesth 86:1–3
Kamibayashi T, Maze M (2000) Clinical uses of alpha2-adrenergic agonists. Anesthesiology 93:1345–1349
Herbert MK, Roth-Goldbrunner S, Holzer P, Roewer N (2002) Clonidine and dexmedetomidine potently inhibit peristalsis in the Guinea pig ileum in vitro. Anesthesiology 97:1491–1499
James AN, Ryan JP, Parkman HP (2004) Effects of clonidine and tricyclic antidepressants on gastric smooth muscle contractility. Neurogastroenterol Motil 16:143–153
Stieger DS, Cantieni R, Frutiger A (1997) Acute colonic pseudoobstruction (Ogilvie's syndrome) in two patients receiving high dose clonidine for delirium tremens. Intensive Care Med 23:780–782
Fruhwald S, Scheidl S, Toller W, Petnehazy T, Holzer P, Metzler H, Hammer HF (2000) Low potential of dobutamine and dopexamine to block intestinal peristalsis as compared with other catecholamines. Crit Care Med 28:2893–2897
Dive A, Foret F, Jamart J, Bulpa P, Installe E (2000) Effect of dopamine on GI motility during critical illness. Intensive Care Med 26:901–907
Lobo DN (2004) Fluid, electrolytes and nutrition: physiological and clinical aspects. Proc Nutr Soc 63:453–466
Lobo DN, Bostock KA, Neal KR, Perkins AC, Rowlands BJ, Allison SP (2002) Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet 359:1812–1818
Nisanevich V, Felsenstein I, Almogy G, Weissman C, Einav S, Matot I (2005) Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology 103:25–32
Golzarian J, Scott HW Jr, Richards WO (1994) Hypermagnesemia-induced paralytic ileus. Dig Dis Sci 39:1138–1142
Behm B, Stollman N (2003) Postoperative ileus: etiologies and interventions. Clin Gastroenterol Hepatol 1:71–80
Dou Y, Gregersen S, Zhao J, Zhuang F, Gregersen H (2001) Effect of re-feeding after starvation on biomechanical properties in rat small intestine. Med Eng Phys 23:557–566
Stacher G (2003) Gut function in anorexia nervosa and bulimia nervosa. Scand J Gastroenterol 38:573–587
Schaefer DC, Cheskin LJ (1998) Constipation in the elderly Am Fam Physician 58:907–914
Booth C, Heyland D, Paterson W (2002) Gastrointestinal promotility drugs in the critical care setting: A systemic review of the evidence. Crit Care Med 30:1429–1435
Delaney CP (2004) Clinical perspective on postoperative ileus and the effect of opiates. Neurogastroenterol Motil 16:61–66
Thompson JS, Quigley EM (1999) Prokinetic agents in the surgical patient. Am J Surg 177:508–514
Prather CM, Camilleri M, Zinsmeister AR, McKinzie S, Thromforde G (2000) Tegaserod accelerates orocecal transit in patients with constipation-predominant irritable bowel syndrome. Gastroenterology 118:463–468
Banh HL, MacLean C, Topp T, Hall R (2005) The use of tegaserod in critically ill patients with impaired gastric motility. Clin Pharmacol Ther 77:583–586
Galligan JJ, Vanner S (2005) Basic and clinical pharmacology of new motility promoting drugs. Neurogastroenterol Motil 17:643–653
Chapman MJ, Fraser RJ, Kluger MT, Buist MD, De Nichilo DJ (2000) Erythromycin improves gastric emptying in critically ill patients intolerant of nasogastric feeding. Crit Care Med 28:2334–2337
Fruhwald S, Herk E, Hammer HF, Holzer P, Metzler H (2004) Differential reversal of drug-induced small bowel paralysis by cerulein and neostigmine. Intensive Care Med 30:1414–1420
Paran H, Silverberg D, Mayo A, Shwartz I, Neufeld D, Freund U (2000) Treatment of acute colonic pseudo-obstruction with neostigmine. J Am Coll Surg 190:315–318
Abeyta BJ, Albrecht RM, Schermer CR (2001) Retrospective study of neostigmine for the treatment of acute colonic pseudo-obstruction. Am Surg 67:265–268
Spoel JI van der, Oudemans-van Straaten HM, Stoutenbeek CP, Bosman RJ, Zandstra DF (2001) Neostigmine resolves critical illness-related colonic ileus in intensive care patients with multiple organ failure -- a prospective, double-blind, placebo-controlled trial. Intensive Care Med 27:822–827
Villar HV, Norton LW (1979) Massive cecal dilation: pseudoobstruction versus cecal volvulus? Am J Surg 137:170–174
Nematy M, O'Flynn JE, Wandrag L, Brynes AE, Brett SJ, Patterson M, Ghatei MA, Bloom SR, Frost GS (2006) Changes in appetite related gut hormones in intensive care unit patients: a pilot cohort study. Crit Care 10:R1–R9
Trudel L, Tomasetto C, Rio MC, Bouin M, Plourde V, Eberling P, Boitras P (2002) Ghrelin/motilin-related peptide is a potent prokinetic to reverse gastric postoperative ileus in rat. Am J Physiol Gastrointest Liver Physiol 282:G948–952
De Winter BY, Boeclxstaens GE, De Man JG, Moreels TG, Herman AG, Pekckmans PA (1997) Effects of mu- and kappa-opioid receptors on postoperative ileus in rats. Eur J Pharmacol 339:63–67
Delaney CP, Weese JL, Hyman NH, Bauer J, Techner L, Gabriel K, Du W, Schmidt WK, Wallin BA, Alvimopan Postoperative Illeus Study Group (2005) Phase III trial of alvimopan, a novel, peripherally acting, mu opioid antagonist, for postoperative ileus after major abdominal surgery. Dis Colon Rectum 48:1114–1125
Yuan CS, Doshan, Charney MR, O'Connor M, Karrison T, Maleckar SA, Israel RJ, Moss J (2005) Tolerability, gut effects, and pharmacokinetics of methylnaltrexone following repeated intravenous administration in humans. J Clin Pharmacol 45:538–546
Schuurkes JAJ (1999) Pharmacotherapy of gastrointestinal motor disorders. In: Herbert MK, Holzer P, Roewer N (ed) Problems of the gastrointestinal tract in anesthesia, the perioperative period and intensive care. Springer, Berlin Heidelberg New York, pp 12–22
Husebye E, Engedal K (1992) The patterns of motility are maintained in the human small intestine throughout the process of aging. Scand J Gastroenterol 27:397–404
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fruhwald, S., Holzer, P. & Metzler, H. Intestinal motility disturbances in intensive care patients pathogenesis and clinical impact. Intensive Care Med 33, 36–44 (2007). https://doi.org/10.1007/s00134-006-0452-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-006-0452-7