Article Text
Abstract
Background Fontan-associated liver disease is accompanied by a hypercoagulable state. While hepatic dysfunction in Fontan patients is common, its relationship with haemostatic changes and clinical outcomes in this patient population remains unclear.
Objective To correlate liver dysfunction and haemostatic profiles with clinical outcomes in the Fontan population.
Patients/methods Patients were enrolled in a multicentre, cross-sectional study in Australia and New Zealand. Hepatic structure and function were assessed using serum-based calculations (Fibrotest and model for end-stage liver disease excluding international normalised ratio scores). Haemostatic profiles were assessed by Thrombin Generation. Platelet function was assessed via Platelet Factor 4 (PF4) and P-selectin (P-SEL). Clinical outcomes were obtained from the Australian and New Zealand Fontan Registry.
Results Seventy-three patients participated in the study (mean age 18.9±8.5 years with a mean of 13.5±6.9 years post-Fontan). The Endogenous Thrombin Potential (ETP) for patients who suffered thrombotic events (TE) (1366.4±66.2 nM/min) was higher compared with patients with major bleeding events (1011.1±138.4 nM/min) (p=0.03). Except for a negative correlation between Fibrotest-score and PF4 (p=0.045), PF4 and P-SEL concentrations did not correlate with markers of hepatic dysfunction or structural abnormality.
Conclusions Increased ETP is associated with TE during clinical follow-up after Fontan. This study reinforces that hepatic dysfunction may contribute to the derangement of coagulation factors, impacting the individual risk of haemostatic complications for the Fontan population.
- Fontan procedure
- Hemorrhage
- Hemostasis
- Liver cirrhosis
- Thrombosis
Data availability statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information. Data consists of deidentified participant data from the Australian and New Zealand Fontan registry and was registered with consent and within ethical approval within the Fontan Liver Renal study (doi:10.1016/j.ijcard.2018.07.118). Data can be requested by contacting professor VI on verai@unimelb.edu.au.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key questions
What is already known about this subject?
Congestive hepatopathy is a common complication of Fontan physiology. Fontan patients also suffer from increased bleeding and thrombotic complications. The effect of liver disease on haemostasis is a field of interest and recently advances in the understanding of the haemostatic system in liver disease have provided strong support for a tenuous equilibrium of haemostasis which can easily be tipped towards a more bleeding-like or thrombotic-like diathesis. Little research has been done to investigate the effect of congestive hepatopathy on the haemostasis of Fontan patients. There is little evidence available to guide anticoagulant therapy in Fontan patients and current therapy is based on the clinical assessment for anatomic and haemodynamic risk factors and expert opinion
What does this study add?
This is the first large study within this population to investigate haemostatic profiles and to correlate these to hepatic dysfunction and clinical outcome. This study stresses the complexity of haemostasis in liver disease, especially in Fontan patients.
How might this impact on clinical practice?
It might change the approach clinicians have to haemostasis in Fontan population. It also shows promise for thrombin generation to be used as modality to assess the haemostatic profile in Fontan patients and to aid in the stratification of anticoagulant therapy.
Introduction
Since its first application in 1968, the Fontan procedure has undergone significant modifications and has greatly improved the prognosis of patients with single ventricular congenital heart disease.1–5 With the ageing Fontan population, clinicians are now presented with the challenge of managing the haemodynamic and haemostatic changes and the associated long-term complications that follow Fontan sequelae.6 The prevalence of adverse events associated with these changes such as TE and bleeding events is increasing. The prevalence rate of thrombotic events (TE) has been reported to be as high as 33%.7 Currently, no modality is available to assess the risk of bleeding and TE and anticoagulant therapy is given based on clinical assessment for anatomic- and haemodynamic risk factors and expert opinion.2 5
Recently, a hypercoagulable state in Fontan patients was described and several prothrombotic mechanisms have been proposed.3 6 Fontan physiology is prone to significant vascular remodelling contributing to overall cardiac dysfunction.8 Adding to that, the haemodynamic changes in Fontan physiology such as low cardiac output, decreased pulsatile pulmonary blood flow, arrhythmias and heart failure lead to increased central venous pressure which is up to three times higher compared with the pre-Fontan lower body venous pressure.1 3 Fontan-associated congestive hepatopathy (CH) is a common complication of Fontan physiology and leads to histopathologic changes in the liver-tissue and is associated with a derangement in procoagulant and anticoagulant protein levels and activity.1 3
Decreased activity of coagulation factors and protein S has been observed in a representative Fontan population. While these patients have increased levels of liver-derived antithrombin and endothelium derived free tissue factor pathway inhibitor (TFPI), they have a paradoxically increased thrombin generation (TG).6 The increased TG might be explained by the increased platelet activation (higher levels of soluble P-selectin and serum soluble CD40 ligand) as well as endothelial damage (increased von Willebrand Factor). Fontan patients also have a slightly lower inhibition of thrombin formation as reflected by a decrease in protein S, increase in thrombin-activated fibrinolysis inhibitor and plasminogen activator inhibitor-1 antigen concentration. These complex haemostatic disorders may not in the least case be caused by liver disease as is reflected by the enhanced platelet activation and endothelial damage, impaired fibrinolysis and heightened thrombin formation observed in this population.6 9–12
Recent advances in the understanding of the haemostatic system in liver disease have provided strong support for a tenuous equilibrium of haemostasis, which can easily be tipped towards a more bleeding-like or thrombotic-like diathesis due to depletion of both procoagulant and anticoagulant factors, changes in factor activity, vascular changes, endothelial damage, platelet activation and changes in vascular flow.11
All elements of Virchow’s triad are present in the Fontan population and while they have increased concentration of antithrombin and endothelium-derived free TFPI, this could explain why the net effect seems to tip their haemostatic balance towards a more prothrombotic phenotype.6 9–12
Understanding the laboratory measures associated with bleeding- and TE in Fontan physiology might aid in stratifying these patients for early detection of haemostatic derangement and hence carries the potential to implement timely prevention or treatment strategies. The TG curve or Thrombogram is an established tool to assess global coagulable state, as it reflects the overall function of the blood clotting system and its inhibitors.13–16 TG in platelet-poor plasma (PPP) makes it possible to investigate the net effect of changes in procoagulant and anticoagulant factors.14
This prospective cohort study set out to investigate the relationship between clinical outcome and haemostatic profiles and hepatic dysfunction in Fontan patients. The specific study aims were to:
Correlate TG to bleeding and TE; correlate plasma-based markers of platelet activity to bleeding and TE; and correlate liver dysfunction to TG, platelet activity parameters and bleeding and TE.
We hypothesised that: (1) Hepatic dysfunction in Fontan patients leads to significant changes in both procoagulant and anticoagulant factors which results in a more thrombotic phenotype as measured by TG; (2) The combination of both liver dysfunction and increased platelet activation leads to more TE in this population.
Methods
Design
Cross-sectional observational study.
Patients
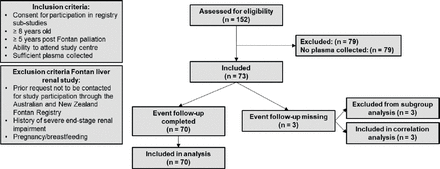
Suitable study subjects for the Fontan Liver Renal study were recruited by using the Australian and New Zealand Fontan Registry, the full design and administration of which has been described previously.17 All 152 included patients in the Fontan Liver Renal study were eligible for inclusion. The Fontan Liver Renal study collected data from three sites located in Melbourne (Victoria, Australia) and Sydney (New South Wales, Australia) and Auckland (New Zealand), as described elsewhere.18 Details regarding patient eligibility and inclusion and exclusion criteria are presented in figure 1.
Flow chart of patient inclusion.
Patient and public involvement
It was not appropriate to involve patients in the design, conduct and reporting of this study.
Laboratory samples
Laboratory tests for the New South Wales, Victorian and New Zealand participants were performed at the Children’s Hospital at Westmead in Sydney, the Murdoch Children’s Research Institute in Melbourne and the Starship Children’s Hospital in Auckland respectively. All laboratory measurements were conducted on minimum 8-hour fasting venous blood samples. Age-dependent variables are presented as percentage of the upper limit of normal based on age and gender.
Hepatic structure and function
This study builds adopts the assessments of hepatic structure and function as reported in the Fontan Liver and Renal study.18 Additionally, the model for end-stage liver disease excluding international normalised ratio (MELD-XI) scores was calculated by using bilirubin and creatinine by use of the following formula: MELD-XI=11.76 (loge creatinine)+5.112 (loge total bilirubin)+9.44.19
Haemostatic profile
The coagulation profile was determined by TG and surrogate platelet activation markers. TG was measured using the Calibrated Automated Thrombinoscope in PPP with 5pM PPP reagents and TG was performed in triplet to minimise intraexperiment variation. TG data are reported in endogenous thrombin potential (ETP), lag time, peak, time to peak, velocity index, start of tail and alpha 2 macroglobulin (α2M) level. Platelet activation was assessed by ELISA determination of platelet factor 4 (PF4 or CXCL4) and soluble P-selectin (sGPVI or CD62P). Plasma PF4 was measured using the abcam PF4 (CXCL4) human SimpleStep ELISA Kit. CD62P was measured using the R&D Systems Human P-Selectin/CD62P Immunoassay.
Clinical outcome
Primary outcome measures were defined as major bleeding, minor bleeding, bleeding and/or TE (table 1). Major bleeding events were defined by a modification of the definition published by the International Society on Thrombosis and Haemostasis.20 Medical history was investigated from the date samples were collected until august 2018. The average duration of follow-up is reported in table 2.
Definition of major bleeding, minor bleeding and thrombotic event
Patient characteristics
Analysis
Data were analysed using IBM SPSS statistics V.25 (IBM). Values reported as being higher or lower than a certain value were analysed as that value for calculation purposes. Continuous variables (eg, all laboratory tests) are expressed as mean and SD or median and range, based on their distribution. Categorical variables (eg, bleeding and thrombosis) are expressed as number and percentage.
To investigate differences in nominal data, a χ2 test for goodness of fit was performed. To assess differences in thrombin-generation-related variables and outcomes between primary outcome groups, an independent-samples t-test was used. A Mann-Whitney U test was used if data was not normally distributed.
To investigate the relationship between measures of haemostasis and other variables correlation analysis was performed considering age, gender and other variables as possible confounders. A scatter plot was made to assess whether the relationship between variables appeared to be linear, if not a Kendall’s τ b analysis was used to test correlation between variables. Trends over time were plotted and analysed for gradient of change.
It has been argued that while interpreting p values, potentially medically important differences observed in small studies, for which the p value is more than 0.05, are simply denoted as non-significant and ignored. Some subgroup analyses within this study are likely to be performed between small groups. To prevent loss of potentially important results p values between 0.05 and 0.10 are reported as trends.
Results
Patient characteristics
A total of 73 patients were included in this study, figure 1. Patient characteristics and characteristics of the clinical event groups are presented in tables 2 and 3. Gender did not influence any of the study variables. Age had a weak positive correlation with lag time (tau=0.204, p=0.016), time to peak (tau=0.274, p=0.001) and start of tail (tau=0.266, p=0.001). A Mann-Whitney U test showed no significant difference in age between subgroups. The intracardiac total Cavo-pulmonary connection-Fontan type (M=2.59, SD=4.72) has a significantly lower MELD-XI score when compared with the atriopulmonary connection (M=6.58, SD=3.38, t (57)=2.413, p=0.019, two tailed, d=0.97) and to the extracardiac total cavopulmonary connection-Fontan type (M=6.21, SD=4.72, t (62)=−2.54, p=0.014, two tailed, d=−0.77). Other study variables did not differ between Fontan Types.
Patient characteristics of the clinical event subgroups
Subgroup analysis
Differences in TG, platelet activity markers and hepatic dysfunction between the no-events group and the different subgroups are presented in table 4. The no-events group (M=156.20, SD=61.32) has a significantly lower velocity index compared with the thrombotic group (M=178.33, SD=10.58), t (−2.24)=12.68, p=0.044, two tailed, d=0.50, 95% CI (–43.57 to –0.69]. There were no significant differences in surrogate platelet activity between the no-events group and the different subgroups. When compared with the no-events group (M=3.31, SD=4.68), the major bleeding group (M=10.1, SD=4.74) has a significantly higher MELD-XI score, t (−2.00)=62, p=0.05 95% CI (–13.47 to –0.01). The minor bleeding group (M=9.23, SD=2.52) also has a significantly higher MELD-XI score compared with the no events group, t (−2.16)=63, p=0.034 95% CI (–11.39 to –0.45). There were no statistically significant differences in Fibrotest-score between the no-events group and the different subgroups.
Comparison of thrombin generation, surrogate platelet activity and hepatic dysfunction outcome between the no-events group and the thrombotic, major bleeding and minor bleeding group
Differences in TG, platelet activity markers and hepatic dysfunction between the TE group and the major- and minor bleeding group are shown in table 5. The thrombotic group (M=1366.39, SD=66.22) has significantly higher ETP than the major bleeding group (M=1011.06, SD=138.44), t (3)=4.03, p=0.027, two tailed, d=3.27 95% CI (74.99 to 635.68). Comparison of the thrombotic group and the minor bleeding group (M=1218.25, SD=74.33) showed a trend for a higher ETP in the thrombotic group, p=0.061. The ETP distribution between subgroups is depicted in figure 2. There were no significant differences in surrogate platelet activity between the TE group and the major-bleeding and minor-bleeding group. The thrombotic group has a significantly lower MELD-XI score than the minor bleeding group, t (−4.48)=4, p=0.011 95% CI (–12.53 to –2.95). There were no statistically significant differences in Fibrotest-score between the TE group and the major-bleeding and minor-bleeding group.
Comparison of thrombin generation, surrogate platelet activity and hepatic dysfunction outcome between the thrombotic-events group and the major-bleeding and minor-bleeding group
Distribution of endogenous thrombin potential between the different subgroups.
Differences in TG, platelet activity markers and hepatic dysfunction between the major-bleeding and minor-bleeding group are shown in table 6. There were no statistically significant differences in TG, platelet activity or hepatic dysfunction between the major-bleeding and the minor-bleeding group.
Comparison of thrombin generation-, surrogate platelet activity and hepatic dysfunction outcome between the major-bleeding and minor-bleeding group
Hepatic dysfunction
The results of the correlation analyses between markers for hepatic dysfunction and TG and surrogate platelet activity are reported in table 7.
Correlation of markers for hepatic dysfunction with thrombin generation and platelet activity markers
There was a weak positive correlation between the MELD-XI score and the start of the tail of the TG curve, τ=0.17, p=0.045. There was a weak positive correlation between MELD-XI score and time to peak, τ=0.207, p=0.013. The platelet activity markers and the remaining TG variables did not correlate with MELD-XI scores.
There was a weak negative correlation between the Fibrotest score and PF4, τ=−0.20, p=0.046. Other surrogate markers for platelet activity and TG variables did not correlate with Fibrotest score.
Discussion
An increased ETP was found in the thrombotic group compared with the major-bleeding group and the thrombotic group showed a trend for a higher ETP compared with the minor bleeding group. An increased ETP has been correlated to a more coagulable state which is associated with an increased risk of TE.21 The mean ETP of the no-events group was not statistically different from the thrombotic- and the major-bleeding group which might be due to the small sample sizes. The mean ETP of the no-events group did, however, lie between the mean values of the thrombotic and bleeding groups. This limits the use of TG as a predictive test for haemostatic complications but shows potential for its use in the early detection of haemostatic derangements and stratification of anticoagulant therapy.
The height of peak showed a trend for a higher peak in the thrombotic group which lead to a more hypercoagulable state. The height of peak shows the highest amount of active thrombin at a certain time. A lower peak is associated with a more bleeding like diathesis.22 When compared with the no events group, the thrombotic group has a higher velocity index while no significant difference was observed between the thrombotic group and the bleeding groups. There was a trend for a higher α2M level in the minor bleeding group compared with the no-events group and a trend for a lower α2M level in the major bleeding group compared with the thrombotic group. α2M functions as an inhibitor of fibrinolysis and thrombin but the direction of effect of the observed α2M levels on thrombus formation is however unclear.23 A higher velocity index reflects the speed at which prothrombin is converted to thrombin.21 It is possible that the higher velocity index in the thrombotic group is the reason these patients are more likely to develop thrombosis.
An inverse correlation was observed between MELD-XI score and time to peak and the start of the tail of the TG curve. The start of the tail is the moment at which there is no free thrombin and that at which thrombin only exists as α2M-thrombin complex.14 Our finding, therefore, suggests that hepatic dysfunction might lead to a longer time to peak, which could be explained by the derangement in procoagulant and anticoagulant factors which has been reported within this patient population. A longer time to peak is however associated with a bleeding like phenotype. A later start of the TG curve tail might be explained by a longer time to peak but could also be explained by a slower inactivation of free thrombin by α2M. This would allow thrombin to be active for a longer time and could thereby lead to a more thrombotic-like diathesis.
The observed differences in MELD-XI scores between subgroups might have arisen due to differences in prevalence of different Fontan types since Fontan types were not evenly distributed between the different subgroups. Concerns about which modality to use to determine liver dysfunction in CH, as seen in Fontan patients, have been raised.1 18 Current clinical tools as serum tests, imaging studies, liver stiffness measurements and liver biopsy failed to accurately estimate hepatic fibrosis or the risk for hepatic decompensation in Fontan patients. Wilson et al published data demonstrating changes in several different non-invasive markers of liver and renal damage in Fontan patients but also acknowledges the limits of using such modalities in this population.18 Even liver biopsy, considered the golden standard to assess fibrosis staging, has little predictive value due to the heterogeneous collagen depositions observed in CH. Conjointly, liver biopsy carries a significant bleeding risk and is therefore seldomly performed. At this moment, the MELD-XI score is the only validated modality to assess hepatic dysfunction and predict clinical outcome in CH but its correlation to clinical outcome is moderate at best making it far from the perfect method to study liver fibrosis in this population.1 Staging hepatic dysfunction in this patient group remains challenging and highlights the need for care in data interpretation. Prescribing anticoagulants to Fontan patients is elaborate considering the disturbances in the haemostatic system and the far-reaching consequences of both bleeding and TE. Scientific Statements from the American Heart Association, American College of Chest Physician Guidelines and European Society of Cardiology Guidelines on the management of grown-up congenital heart disease state that thromboprophylaxis post-Fontan surgery is appropriate considering the high risk of thrombosis associated morbidity and mortality.24–26 Current evidence for the optimal antithrombotic therapy and duration for Fontan patients is limited.27 Importantly, bleeding complications do occur in Fontan patients and that risk is increased by antithrombotic therapy.2 These results show potential for the clinical application of TG as modality to stratify anticoagulant therapy for post-Fontan patients.
Limitations
This study is limited by the inherent design of observational research and the noted shortfalls of doing research in this specific population. In pointing out the limitations of this study, we acknowledge that some of the reported trends might have arisen as statistical consequence of the small sample sizes within various subgroups and should therefore be interpreted with caution.
Conclusions
This is the first large study to investigate the haemostatic profile in Fontan patients with hepatic dysfunction and to correlate their haemostatic profiles to bleeding and TE. We found that ETP is associated with TE and major-bleeding events during clinical follow-up after Fontan palliation. This study underlines the importance of taking a broader approach to haemostasis in patients with a Fontan circulation and hepatic dysfunction, as their physiology greatly affects most factors within the haemostatic system.
Data availability statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information. Data consists of deidentified participant data from the Australian and New Zealand Fontan registry and was registered with consent and within ethical approval within the Fontan Liver Renal study (doi:10.1016/j.ijcard.2018.07.118). Data can be requested by contacting professor VI on verai@unimelb.edu.au.
Ethics statements
Ethics approval
The full design of the present study has been approved by the national and local ethics committees of all participating hospitals.
Acknowledgments
The authors acknowledge support provided to the Murdoch Children’s Research Institute by the Victorian Government’s Operational Infrastructure Support Programme. Yves d'Udekem is a NHMRC Clinician Practitioner Fellow (1082186).
References
Footnotes
Twitter @drwinlaw
Contributors JJND: primary investigator. CA: study coordinator, manuscript review. SVDH: ELISA testing. JB: manuscript planning and review. YD: principal investigator. KdP: Fontan Registry employee. TGW: data retrieval and collation, manuscript review. DW: patient recruitment, registry management, clinical review, manuscript review. PM: secondary study supervisor. VI: primary supervisor.
Funding This project was supported by a grant from the National Health and Medical Research Council (NHMRC Project Grant 1047923).
Competing interests YD is consultant for MSD and Actelion. The remaining authors have no conflicts of interest to declare.
Provenance and peer review Not commissioned; externally peer reviewed.