Article Text
Statistics from Altmetric.com
- st elevation myocardial infarction
- cineangiography
- takotsubo cardiomyopathy
- heart rupture
- cardiac tamponade
Part 1
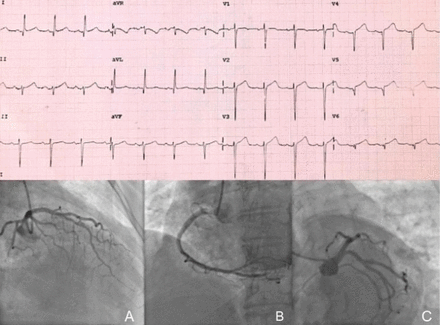
A woman presented to the emergency room complaining resting chest pain, dyspnoea, cold sweating and nausea several hours after symptoms onset. Of note, she had some risk factors for coronary artery disease. She also had been recently notified of a bereavement. On arrival, she was in pain, her vitals were heart rate 90 bpm, respiratory rate 20 rpm, blood pressure 90/60 mm Hg and O2 saturation 91%. Admission ECG is shown in figure 1. She was moved emergently to the catheterisation laboratory while being started on 300 mg aspirin, 600 mg clopidogrel, 5000 U of heparin and 80 mg atorvastatin. Soon, her blood pressure dropped to 60/40 mm Hg and she was started on vasopressors and intravenous fluid resuscitation achieving a blood pressure of 90/60 mm Hg. Coronary angiography is shown in figure 1.
Patient’s admission ECG and coronary angiography.
According to your diagnostic suspicion, what would you do next?
Aortography from the aortic root.
Intravascular ultrasound (IVUS) of the left anterior descending (LAD) coronary artery.
Left ventriculography.
LAD primary angioplasty.
Part 2
Coronary angiography revealed no evidence of a proximal fixed coronary lesion (figure 1, bottom) but likely vasospasm of the LAD (figure 1A). In the left anterior oblique (LAO) projection, pericardial effusion was presumed due to the image of a 2 cm space between the epicardial coronary arteries and the outer border of the heart (figure 1C). Left ventricular end-diastolic pressure pressure was 23 mm Hg. Finally, left ventriculography was performed (figure 2) and showed hyperkinetic basal segments but anteroapical dyskinesia (figure 2D,E) (takotsubo apical variant).1 Contrast media appeared leaking into the pericardial space passing through the apical wall by way of a fistulous tract and collecting outside of it (online supplementary file 1).
Supplementary video
Patient’s left ventriculography showing a typical takotsubo’s apical ballooning with also a left ventricular free-wall rupture causing the patient’s heart tamponade.
The patient’s clinical profile, symptoms, mild troponin elevation and ECG findings (aVR ST segment depression, anterolateral ST segment elevation), in the absence of occlusive coronary disease but LAD spasm, suggested the diagnosis of takotsubo cardiomyopathy.2–4 The later cardiogenic shock and picture (figure 2) were explained by the apical wall rupture.1
An aortography was not indicated because there was no suspicion of aortic pathology. LAD IVUS would have delayed the proper diagnosis in the context of a supposed exclusive LAD spasm. Although coronary spasm and takotsubo cardiomyopathy were once considered separate entities, there is convincing evidence of a common pathophysiology5 6 and there are case reports with this provoked6 and spontaneous7 association. LAD primary angioplasty was not indicated because there was no evidence of acute coronary thrombosis.
Pericardial effusion puncture with blood autotransfusion was attempted while emergent call to cardiovascular surgery team was being made. The haemodynamic collapse was so abrupt that quickly the patient evolved to cardiac arrest refractory to cardiopulmonary resuscitation manoeuvres. There is a case report in which percutaneous closure of an apical free wall rupture with an Amplatzer occluder was attempted as a bridge therapy to an urgent surgical repair, but the patient finally died after the surgical repair attempt.8
Acknowledgments
Alejandro Díaz-Cabañas.
Footnotes
Collaborators Alejandro Díaz-Cabañas.
Contributors All the authors have contributed to the planning, conduct and reporting of the work described in the article.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not obtained.
Provenance and peer review Not commissioned; externally peer reviewed.