Article Text
Abstract
Objectives This study aims to determine the proportion of real-world patients with myocardial infarction (MI) who would have been eligible for the PEGASUS-TIMI 54 (Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis in Myocardial Infarction 54) trial, to characterise their current use of P2Y12 inhibitors and to explore the estimated costs and ischaemic event consequences of increasing P2Y12 inhibitor use among these patients.
Methods In the US national ACTION Registry–GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry–Get With The Guidelines), we identified 273 328 patients with MI and determined the proportion that would have met the eligibility criteria for the PEGASUS trial. We described longitudinal P2Y12 inhibitor use among patients eligible for PEGASUS and estimated the cost and ischaemic consequences of increasing P2Y12 use among eligible patients.
Results A total of 112 222 (41.1%) patients with MI in ACTION Registry–GWTG met eligibility for the PEGASUS trial. Among 83 871 eligible patients with pharmacy claims data, 23 042 (27.5%) were on a P2Y12 inhibitor at 1 year, 9661 (11.5%) at 2 years and 5246 (6.3%) at 3 years, with the majority (79.2%) of these patients on clopidogrel. The use of ticagrelor in eligible patients not yet on a P2Y12 inhibitor at 1 year post-MI would cost an estimated US$885 000 per MI, stroke or cardiovascular death averted over a 3-year time horizon, while the use of clopidogrel would cost an estimated US$19 800 per ischaemic event averted.
Conclusion In contemporary clinical practice, a minority of patients are on a P2Y12 inhibitor beyond 1-year post-MI. Applying PEGASUS trial findings to clinical practice would result in a large increase in P2Y12 inhibitor use, with a cost per ischaemic event averted that is strongly influenced by the choice of therapy.
- P2Y12
- myocardial infarction
- secondary prevention
- cost
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Key questions
What is already known about this subject?
The PEGASUS-TIMI 54 (Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis in Myocardial Infarction 54) trial demonstrated a reduction in ischaemic events following P2Y12 inhibition with ticagrelor among patients with a prior myocardial infarction (MI). The implications of these findings for clinical practice are unknown.
What does this study add?
Less than half of all stable patients with post-MI in routine US clinical practice met the PEGASUS eligibility criteria. Furthermore, P2Y12 inhibitors were infrequently used in the long-term management of patients with MI in the ACTION Registry–GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry–Get With The Guidelines). The estimated cost per ischaemic event averted with increasing P2Y12 inhibitor use is nearly 45-fold larger with long-term ticagrelor than with generic clopidogrel.
How might this impact on clinical practice?
Applying PEGASUS trial findings to clinical practice would result in a large increase in P2Y12 inhibitor use. Given the cost implications for different P2Y12 inhibitors, additional study of long-term P2Y12 inhibitors on patient outcomes and cost of care is needed to guide optimal use of this therapy in clinical practice.
Introduction
The activated platelet is a key contributor to the pathobiology of acute coronary events.1 Dual antiplatelet therapy (DAPT) with aspirin, and a P2Y12 inhibitor has been shown to reduce the risk of ischaemic events in patients with an acute coronary syndrome (ACS).2 The benefit of long-term P2Y12 therapy for patients with stable cardiovascular disease is less clear. A randomised trial of long-term clopidogrel on background aspirin therapy did not demonstrate benefit in a broad population at risk of atherosclerotic events.3 However, post hoc analyses suggested the addition of clopidogrel to aspirin resulted in benefit for certain patient subgroups, including those with a prior history of myocardial infarction (MI).4 These findings implicated potential benefit of long-term DAPT in specific subgroups of patients at high risk of ischaemic events.5–7
Newer generation P2Y12 receptor antagonists are characterised by greater platelet inhibition, less variability in clinical response, and superior efficacy compared with clopidogrel following an ACS.8 9 The recent PEGASUS-TIMI 54 (Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis in Myocardial Infarction 54) trial sought to determine whether the benefit of ticagrelor, a newer generation P2Y12 inhibitor, extends to stable patients with a remote history of MI.10 The study found that ticagrelor use, with background aspirin therapy, resulted in a 1.2% absolute reduction in the rate of cardiovascular death, MI or stroke over 3 years compared with aspirin alone.11
Although the PEGASUS trial findings suggest an intriguing opportunity to improve outcomes of patients with prior MI, patients seen in routine clinical practice may differ from those enrolled in clinical trials.12 Consequently, the prevalence of stable patients with MI in US clinical practice who would meet PEGASUS enrolment criteria for long-term therapy with P2Y12 therapy is unknown. In addition, given the prior post hoc analysis of long-term therapy with clopidogrel4 and recent data demonstrating benefit to prolonged P2Y12 following PCI,13 many patients with a remote history of MI may already be on some form of long-term P2Y12 inhibitor. Finally, clopidogrel became available as a generic prescription in May, 2012, while ticagrelor remains on patent until 2018. As a result, there may be cost differences in consideration of long-term P2Y12 inhibitor use.
Answers to these questions can be measured using clinical registries, such as the American College of Cardiology (ACC) National Cardiovascular Disease Registry (NCDR) programs. The ACC has recently launched the Research to Practice initiative (formerly titled Rapid Registry Response), which facilitates rapid analysis of registry data to understand how clinical trials, such as PEGASUS, inform clinical practice.14 Accordingly, we used this initiative and the NCDR ACTION Registry to determine the proportion of real-world patients with post-MI who would be eligible for the PEGASUS trial, characterise their use of P2Y12 inhibitors and model the costs and ischaemic clinical consequences of adding long-term P2Y12 therapy in accordance with the PEGASUS trial.
Methods
Data source
The NCDR ACTION Registry–GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry–Get With The Guidelines) is a US national quality improvement registry of ST-segment elevation MI (STEMI) and non-STEMI (NSTEMI) patients.15 Trained staff extracts data from medical records into a Web-based case record without direct patient contact. A listing of specific data fields and their definitions is available (http://www.ncdr.com/WebNCDR/ACTION/Elements.aspx). Our study cohort was derived from patients presenting with their first MI in the registry from 1 October 2010 to 30 April 2013 at ACTION Registry–GWTG participating hospitals.
To identify long-term use of DAPT, data from ACTION Registry–GWTG were linked with pharmacy claims data from Symphony Health Solutions, a multichannel claims data resource that includes all payer types, including cash self-pay, and covers pharmacy activity in all 50 US states. The data warehouse captures dispensed prescription drugs from claims clearing-houses, retail pharmacies and prescription benefit managers for approximately 70% of US retail pharmacy transactions. Records comprise National Council for Prescription Drug Program formatted claims, which include the date of a given pharmacy fill, national drug code, drug name and dosage, as well as other standardised fields.16
Patient population
We replicated the inclusion and exclusion criteria from the PEGASUS-TIMI 54 trial to identify patients for whom the findings of this trial may apply.10 11 Accordingly, we identified all patients with an index MI during the study period and then restricted our cohort to patients in the ACTION Registry–GWTG who were 65 years or older at the time of their index MI or who were aged 50 or older at the time of the index MI with one or more additional risk factors of prior MI, diabetes mellitus on oral medications or insulin, or known multivessel coronary disease. Although PEGASUS also included patients aged 50 or older with an additional risk factor of non-end-stage chronic renal dysfunction, ACTION Registry–GWTG data are insufficiently granular to identify patients with this additional risk factor.
In accordance with PEGASUS enrolment criteria, we excluded patients receiving chronic anticoagulation at discharge from the index MI and patients with prior stroke, coronary artery bypass grafting (CABG) in the past 5 years or renal failure requiring dialysis. The ACTION Registry–GWTG lacks data for several comorbid conditions that were exclusion criteria for PEGASUS. As a result, we could not identify patients with a history of gastrointestinal bleed in the past 6 months, surgery in the past 30 days, central nervous system tumour, intracranial vascular abnormality, prior intracranial haemorrhage or severe liver disease that would have been excluded from the PEGASUS trial.
The final analytic cohort included patients eligible for the PEGASUS trial with at least 3 years of prescription claims after the cohort eligibility date.
Use of P2Y12 inhibitors 1 year post-MI
Patients were categorised as users of a P2Y12 inhibitor at 1 year post-MI if they had a pharmacy claim for a P2Y12 inhibitor in the first 3 months that followed 1 year from the index MI. This approach was repeated in years 2 and 3 to establish continued use of P2Y12 inhibitors. Because aspirin is available over the counter, the concurrent use of aspirin could not be reliably confirmed.
Statistical analysis
We identified patients from the ACTION Registry–GWTG who met PEGASUS inclusion and exclusion criteria and compared the cohort with patients from the PEGASUS trial. Among patients eligible for PEGASUS in the ACTION Registry–GWTG, we then compared patient demographics (age, sex, race/ethnicity and insurance status), comorbidities (hypertension, hyperlipidaemia, diabetes, tobacco use, prior MI, prior percutaneous coronary intervention (PCI), prior CABG, prior heart failure, cerebrovascular disease, chronic kidney disease, chronic lung disease and anaemia), clinical presentation (STEMI or NSTEMI), management (PCI, CABG or medical management alone) and estimated bleeding risk, by the use of P2Y12 inhibitors at 1 year after the index MI. Bleeding risk was estimated through the application of a previously validated model from the ACTION Registry–GWTG.17 We then assessed the current use of P2Y12 inhibitors at 1, 2 and 3 years post-MI in our cohort and the specific P2Y12 inhibitors used. Cohort differences were assessed using analysis of variance for continuous variables and χ² test for categorical variables.
In the cost and ischaemic clinical consequences analysis, we first determined the cost of increasing the use of P2Y12 inhibitors in the eligible cohort. We estimated the cost of placing all patients eligible for PEGASUS who were not on a P2Y12 inhibitor at year 1 post-MI on ticagrelor for a 3-year time horizon, as reported in the clinical trial. The cost of ticagrelor was estimated based on the Wholesale Acquisition Cost. Generally, Wholesale Acquisition Cost is the price established by the drug manufacturer before any rebates, discounts, allowances or other price concessions are offered by the supplier of the product. We also estimated the cost of clopidogrel therapy in all patients eligible for PEGASUS who were not on a P2Y12 inhibitor at year 1 post-MI using the same methods.
To estimate the ischaemic clinical consequences of placing all patients eligible for PEGASUS who were not on a P2Y12 inhibitor at year 1 post-MI on ticagrelor, we applied the point estimate for the clinical effect reported in the PEGASUS trial over a 3-year time horizon.11 We estimated the number of prevented cardiovascular events due to ticagrelor therapy by multiplying eligible patients by the average effect size for cardiovascular events reported in the PEGASUS trial (1.27% 3-year absolute risk reduction). Because a randomised comparison of the clinical effect of clopidogrel versus ticagrelor in stable patients with post-MI has yet to be completed and the point estimate for the clinical effect of clopidogrel in a post hoc analysis of the CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance) trial (30-month absolute risk reduction of 1.7%) is larger than that reported for ticagrelor in PEGASUS, we estimated the clinical consequences of adding clopidogrel therapy by applying the same point estimate used in our base case evaluation of ticagrelor.4 Clopidogrel was chosen for this analysis given prior studies of this therapy in similar clinical settings to the PEGASUS trial and because clopidogrel is available as a generic medication. In sensitivity analyses, we varied the estimated clinical efficacy of these P2Y12 therapies by 25% above and below the point estimate from the randomised trials to provide a range of the potential cost consequences of expanding therapy. We applied similar methods in the estimation of additional bleeding events related to increased ticagrelor use (1.24% 3-year absolute bleeding risk increase with ticagrelor 60 mg in the PEGASUS trial).
Results
We identified 273 328 patients with an index MI in the ACTION Registry–GWTG during the period of study. After applying inclusion and exclusion criteria from the PEGASUS trial, 112 222 (41.1%) of these patients met enrolment criteria for the trial (figure 1). We were able to match 83 871 (74.7%) of these patients to Symphony pharmaceutical claims data to assess P2Y12 inhibitor use between 1 and 3 years post-MI. Characteristics for patients for the PEGASUS trial randomised to 60 mg ticagrelor are descriptively compared with patients eligible for PEGASUS in the ACTION Registry–GWTG in table 1 without statistical tests of significance, given the lack of patient-level data from the PEGASUS trial for the present study. Patients eligible for PEGASUS in the ACTION Registry–GWTG appeared to be older, were more often female, less often presented with a STEMI and were more likely to suffer an MI or stroke in the 3-year follow-up.
Identification of patients eligible for PEGASUS in the ACTION Registry–GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry–Get With The Guidelines) and analysis cohort. SHS, Symphony Health Solutions.
Comparison of patients eligible for the PEGASUS (60 mg ticagrelor with patients eligible for PEGASUS identified from the ACTION Registry-GWTG
At 1-year post-MI, 23 042 (27.5%) of patients eligible for PEGASUS in the ACTION Registry–GWTG were on a P2Y12 inhibitor. The majority of patients on a P2Y12 inhibitor were on clopidogrel (79.2%), followed by prasugrel (17.7%) and ticagrelor (3.0%). Patient characteristics by use of P2Y12 inhibitors at 1 year post-MI are shown in table 2. Given the size of our cohort, many differences were statistically significant, although the absolute differences were generally small, with some exceptions. Compared with patients who were not on a P2Y12 inhibitor at 1 year post-MI, patients on a P2Y12 inhibitor were more likely to have private insurance (30.7% vs 23.2%, p <0.001) or received PCI with a drug-eluting stent (65.5% vs 36.9%) and less likely to have prior MI (16.5% vs 19.7%, p <0.001) or anaemia (17.9% vs 24.6%). Compared with patients who were not on a P2Y12 inhibitor at 1 year, estimated bleeding risk was slightly lower among patients on P2Y12 therapy (moderate risk 27.5% vs 30.1%, high risk 2.4% vs 4.0%; p <0.001).
Characteristics of patients eligible for PEGASUS in the ACTION Registry–GWTG by P2Y12 use 1 year after MI
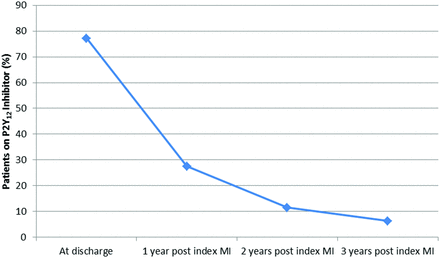
The number of patients on a P2Y12 inhibitor declined over time to 9661 (11.5%) at 2 years post-MI and 5246 (6.3%) at 3 years post-MI (figure 2). Characteristics of patients on a P2Y12 inhibitor at 1, 2 and 3 years post-MI were largely similar (table 3).
Proportion of patients eligible for PEGASUS in the ACTION Registry–GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry–Get With The Guidelines) on P2Y12 inhibitors over time. MI, myocardial infarction.
Patients eligible for PEGASUS in the ACTION Registry–GWTG characteristics by the use of a P2Y12 inhibitor at 1, 2 and 3 years post-MI
The Wholesale Acquisition Cost for a 1-year supply of 60 mg ticagrelor two times per day was US$3750. The estimated cost of initiating and continuing ticagrelor for a 3-year time horizon in 60 829 patients who were eligible for the PEGASUS trial but not on a P2Y12 inhibitor at 1-year post-MI was US$684 million. The estimated number of cardiovascular deaths, MI or strokes averted over a 3-year time horizon by initiation of ticagrelor in this population was 773, which translates into a cost of US$885 000 per cardiovascular event averted. In the sensitivity analysis, when the estimated clinical efficacy was varied by 25% above and below the point estimate reported in the PEGASUS trial, the cost per cardiovascular event averted ranged from US$709 000 to US$1.2 million. The estimated number of additional bleeding events over a 3-year time horizon following initiation of ticagrelor in this population was 754.
The Wholesale Acquisition Cost for a 1-year supply of 75 mg generic clopidogrel daily was US$84, with an estimated total cost of US$15.3 million to initiate and continue clopidogrel in the same population of 60 829 patients for a 3-year time horizon. Estimating a similar number of cardiovascular deaths, MI or strokes averted as would be achieved with ticagrelor over a 3-year time horizon, this translates into US$19 800 per cardiovascular event averted. In the sensitivity analysis, when the estimated clinical efficacy was varied by 25% above and below the point estimate, the cost per cardiovascular event averted ranged from US$15 800 to US$26 400.
Discussion
We assessed the implications of the PEGASUS-TIMI 54 trial in a real-world population of patients drawn from the ACTION Registry–GWTG, including the number of eligible patients, current use of P2Y12 inhibitors and the cost and ischaemic consequences of long-term P2Y12 inhibition. Among 273 328 patients with MI in the ACTION Registry–GWTG, 112 222 (41.1%) met eligibility for the PEGASUS trial. Among patients eligible for PEGASUS, only 27.5% were on a P2Y12 inhibitor at 1 year post-MI. Initiating and continuing ticagrelor in patients eligible for PEGASUS who were not yet on a P2Y12 inhibitor at 1-year post-MI would cost an estimated US$885 000 per MI, stroke or death averted over a 3-year time horizon. The cost per ischaemic event averted was much lower for generic clopidogrel. These findings suggest that P2Y12 inhibitors are not routinely used in long-term care of patients with a prior history of MI. Adoption of the PEGASUS strategy in clinical practice would result in a large increase in P2Y12 use, and the cost per cardiovascular event averted would vary dramatically with the choice of P2Y12 inhibitor.
Evidence supporting long-term P2Y12 use in patients with prior myocardial infarction
Although a number of studies have investigated the long-term use of DAPT after PCI, investigation of the clinical efficacy of DAPT in long-term management of patients with a prior MI is more limited. The CHARISMA trial failed to demonstrate a clinical benefit from long-term clopidogrel use when added to aspirin in a broad population of patients with elevated cardiovascular risk.3 However, a subgroup analysis of this trial suggested a potential benefit of P2Y12 inhibitor use among patients with a prior history of MI.4 These findings were supported by the PEGASUS-TIMI 54 trial, in which the addition of ticagrelor to background aspirin therapy resulted in a 1.27% reduction in the rate of cardiovascular death, MI or stroke among patients with a remote history of MI and an additional risk factor.7 In both studies, the addition of long-term P2Y12 inhibition was not associated with a reduction in all-cause mortality. However, given the implications for ischaemic events, clinical practice guidelines state the continuation of DAPT may be reasonable among patients with a prior MI between 1 and 3 years earlier who have tolerated DAPT without bleeding complications.6
Applicability of the PEGASUS trial to real-world practice
Despite the potential benefit of long-term P2Y12 inhibitors therapy in patients with prior MI, the number of patients who might benefit is unknown. The population evaluated in clinical trials may not represent the population of patients in clinical practice. In the present study, less than half of all patients with MI met the PEGASUS eligibility criteria. These findings are consistent with prior studies that have demonstrated the differences between patients represented in clinical trials and patients seen in clinical practice.12
Indiscriminate use of P2Y12 inhibitors among patients with a prior MI may result in the treatment of a lower risk population than represented by the PEGASUS trial and, potentially, a smaller effect on ischaemic events. In the present study, one in five patients with prior MI was not PEGASUS eligible, because the patient lacked an additional high-risk feature for ischaemic events. Furthermore, nearly 14% of patients were ineligible, because they were on an anticoagulant. The use of P2Y12 inhibitors in these patients may result in significantly higher bleeding risk, which outweighs the ischaemic benefit of P2Y12 inhibitors. These findings highlight the importance of close scrutiny of eligibility as it applies to individual patients in routine practice.
Long-term use of P2Y12 inhibitors after myocardial infarction in clinical practice
P2Y12 inhibitors were infrequently used in the long-term management of patients with MI in the ACTION Registry–GWTG. It will be important to study the potential changing use of this therapy over time to understand the clinical effectiveness and safety of P2Y12 inhibitors in real-world settings, both among patients who meet eligibility criteria for the randomised trials of this therapy and among the more than 50% of patients with prior MI who are not represented in the randomised trials of this therapy. In addition, our study suggests that non-clinical factors such as insurance status influences the use of P2Y12 in current practice. Identifying and addressing barriers to the use of P2Y12 inhibitors will be important to guide optimal use in eligible patients. Further, we found patients at higher bleeding risk were less likely to receive long-term P2Y12 inhibitors in current practice. This is consistent with the thoughtful use of P2Y12 inhibitors, as a trade-off between decreasing ischaemic risk and increasing bleeding risk is intrinsic to the long-term use of P2Y12 inhibitors. Ensuring P2Y12 inhibitors are used in patients with higher predicted ischaemic benefit and lower bleeding risk may further ensure optimal patient outcomes with this therapy in clinical practice.7 18
Although the focus of our study relates to the implications of increasing the use of DAPT beyond 1 year post-MI, it is worth noting that 23% of patients in the ACTION-GWTG Registry did not receive a P2Y12 inhibitor at the time of discharge. This gap in care delivery is consistent with prior studies19–21 and suggests an opportunity to improve patient outcomes through more consistent use of P2Y12 at discharge for MI.
Cost and clinical consequences of long-term P2Y12 inhibitor use
Although the PEGASUS trial specifically evaluated ticagrelor, the CHARISMA substudy suggests a potential class effect of long-term P2Y12 inhibition among patients with prior MI.4 This finding has important implications for the costs of expanding P2Y12 use in clinical practice. Clopidogrel has been available as a generic medication since 2012 and is less expensive than ticagrelor. As a result, the estimated cost per ischaemic event averted differs by nearly 45-fold between the two alternatives. The cost relative to clinical impact of a given therapy is becoming increasingly relevant, as payers apply these considerations in coverage decisions and provider organisations with Accountable Care Organization contracts seek to optimise outcomes at lower cost.22 Based on data from the Healthcare Utilization Project, the median cost of MI admissions in the USA in 2013 was US$15 402, and the median cost of stroke admissions was US$8949.23 With this as a comparator, the cost of additional clopidogrel use would be nearly offset by the cost of averted events, whereas the cost of additional ticagrelor use would be more than the cost of averted events. However, once the cost of bleeding events are considered, both therapies may be more costly than discontinuation of P2Y12 inhibitors. Additional studies from clinical practice are needed to further inform the effectiveness of these two therapies on ischaemic events and the cost consequences of specific P2Y12 inhibitors used in long-term management.
Strengths and limitations
Strengths of this study include the national perspective and pharmacy claims data to evaluate long-term P2Y12 use in clinical practice. Limitations of our study include the lack of detailed clinical data in follow-up from the index MI, thus requiring extrapolation of clinical data at the time of the MI to estimate eligibility for the PEGASUS trial. As a result, our study may underestimate the proportion of patients who would have been excluded from the PEGASUS trial. We were also unable to adjust for important differences in patient selection necessary to perform comparative effectiveness analyses between use and non-use of P2Y12 inhibitors and different P2Y12 inhibitors. We lacked data on some comorbid conditions that were exclusion criteria for the PEGASUS trial. As a result, our study may overestimate the proportion of real-world patients who would have met eligibility criteria for the PEGASUS trial. We were unable to match 25% of patients eligible for PEGASUS to pharmacy claims data. Our study is limited by a time period that precedes the publication of the PEGASUS trial in 2014. However, the intent of our study was to describe how clinical practice might be influenced by this study, and future studies are needed to understand practice changes in P2Y12 inhibitor use subsequent to the publication of this trial. Although we compared the estimated ischaemic clinical consequences of increasing the use of long-term P2Y12 therapy, increased bleeding is a risk of long-term use of this therapy. However, differences in the reporting of bleeding events across the PEGASUS trial and the CHARISMA substudy limit the ability to extrapolate the estimated impact beyond what has been reported in these trials. We were limited in our evaluation to ischaemic events using point estimates from the randomised controlled trials for the time horizons studied. Further study of P2Y12 therapies in routine clinical practice is needed to further understand the ischaemic benefit and bleeding risk trade-offs of these therapies in real-world use. Finally, we did not attempt to convert clinical consequences to health utilities (ie, quality-adjusted life years), as our intent was to describe the costs and ischaemic clinical consequences of expanding P2Y12 inhibitor use rather than the cost-effectiveness of the therapy.
Conclusion
A minority of patients in the ACTION Registry–GWTG were on a P2Y12 inhibitor beyond 1 year post-MI. Applying PEGASUS trial findings to our patient cohort would result in a large increase in P2Y12 inhibitor use. The estimated cost per ischaemic event averted is nearly 45-fold larger with long-term ticagrelor than with generic clopidogrel. Continued study of long-term P2Y12 inhibition on patient outcomes and cost of care is needed to further guide optimal use of this therapy.
References
Footnotes
Contributors SMB, GPH, PS and TMM worked on the design, analysis, data interpretation and manuscript writing. EJA, SAF, JAW, JAV and AS worked on the design, data interpretation and manuscript writing.
Competing interests GPH serves as a member of the Clinical Excellence Committee for Millennium Health and is an employee of Symphony Health, which has a collaborative agreement with the American College of Cardiology. EJA reports consulting for Merck. JHW serves on the New England Comparative Effectiveness Public Advisory Council, which conducts cost-effectiveness analyses of clinical interventions.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement No additional data available. Requests for ACTION-GWTG Data are managed by the American College of Cardiology.