Article Text
Abstract
Severe coronary artery calcification is one of the greatest challenges in attaining success in percutaneous coronary intervention, limiting acute and long-term results. In many cases, plaque preparation is a critical prerequisite for delivery of devices across calcific stenoses and also to achieve adequate luminal dimensions. Recent advances in intracoronary imaging and adjunctive technologies now allow the operator to select the most appropriate strategy in each individual case. In this review, we will revisit the distinct advantages of a complete assessment of coronary artery calcification with imaging and application of appropriate and contemporary plaque modification technologies in achieving durable results in this complex lesion subset.
- Coronary Stenosis
- Atherosclerosis
- Percutaneous Coronary Intervention
Data availability statement
No data are available.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Coronary artery calcification (CAC) is increasingly common and is associated with high rates of immediate procedural failure and adverse clinical outcomes. Given the advances in calcium modification technologies, and studies showing favourable long-term results, this has resulted in an increase in the number of patients with severe calcium treated percutaneously and with greater success. Furthermore, recent advances in both invasive and non-invasive coronary imaging technologies can be combined with these novel technologies for targeted and precise percutaneous coronary intervention (PCI) approaches. In the present article, we review the contemporary evaluation and management of CAC and the implications for future clinical research and practice.
Effect of CAC on outcomes of PCI
Severe CAC is one of the main causes of procedural PCI failure. CAC may impede the advancement of devices, render the stenosis non-dilatable or instigate PCI-related complications including vessel dissection, perforation or mechanical stent problems (stent underexpansion, distortion, dislodgement or loss). All of these complexities translate to a higher procedural risk in patients with angiography-defined stenosis calcification.1 This has been confirmed in intracoronary imaging studies. A number of intravascular ultrasound (IVUS) studies have reported increased rates of periprocedural myocardial infarction (MI) with severe calcification.2 3 In addition, optical coherence tomography (OCT) studies have also shown that PCI in lesions with spotty calcification, especially when associated with thin cap fibroatheroma, is associated with a higher rate of periprocedural MI.4 5
In a pooled analysis of 6855 patients from the HORIZONS-AMI and ACUITY studies, the presence of moderate–severe calcification on angiography was associated with older age, increased coronary artery complexity, lower final minimal lumen diameter (MLD) higher residual stenosis and high rates of periprocedural complications.6 In the same study, this translated to higher rates of clinical events, including MI, target lesion revascularization (TLR), major adverse cardiac events (MACE) and mortality. A large meta-analysis of seven stent trials confirmed the finding that the presence of severe angiographic calcification was associated with lower rates of complete revascularisation and higher rates of adverse outcomes including MI, repeat revascularisation and death.7
In addition, the presence of calcified nodules (CN) has been shown to have an impact on PCI outcomes, particularly in the acute coronary syndrome (ACS) setting (figure 1). In a study of 657 patients with ACS undergoing IVUS-guided PCI, CN were present in 5.3% of the subjects.8 In this study, the presence of CN was associated with increased MACE, ACS recurrence and TLR, with over 80% of the TLR at the CN lesion driven by its reappearance within the implanted drug-eluting stent (DES). In another study of 288 subjects with ACS who underwent OCT-guided PCI, CN were present in 12% of the lesions. The CN group of this study was associated with smaller minimal stent areas, reduced stent expansion index and higher rates of stent malapposition and stent edge dissections.9 In a recent study by the TACTICS investigators in a study of 700 patients with ACS, the underlying cause based on OCT was a CN in 4% of the cases and these patients had a higher risk of MACE at 1 year.10 11
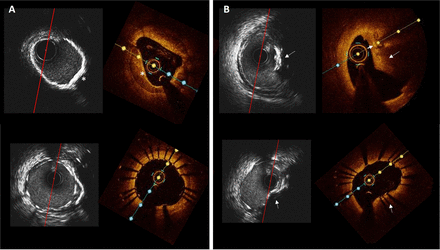
Circumferential versus nodular coronary calcification. (A) Corresponding HD-IVUS and OCT frames of the mid right coronary artery before (above) and after (below) stenting showing a sheet of circumferential calcium (asterisk) with good stent expansion and apposition. (B) Corresponding HD-IVUS and OCT images before (above) and after (below) stenting in the proximal lesion. The stent is expanded but shows an elliptical shape due to the presence of the calcified nodule (arrow). HD-IVUS, high-definition intravascular ultrasound; OCT, optical coherence tomography.
Imaging assessment of coronary calcification: detection, characterisation and guidance for PCI
Invasive coronary angiography
Invasive coronary angiography (ICA) has been the traditional imaging tool to detect CAC. CAC is classified on coronary angiography as moderate severity when radio-opacities are noted only during the cardiac cycle before contrast injection, whereas severe calcification is defined as radio-opacities observed without cardiac motion, and usually affecting both sides of the arterial lumen.12 In addition to its recognised low sensitivity (40–48%),13 14 inherent limitations in its spatial resolution and its inability to depict the arterial wall has led to incorporation of other adjunctive imaging modalities. CT, IVUS and OCT now complement ICA in the evaluation of CAC15 (figure 2 and online supplemental table 1).
Supplemental material
Coronary calcification depicted with multimodality imaging. (A) Angiographic image showing heavily calcified left anterior descending artery (LAD). (B) CT showing widespread calcification. (C) OCT images showing deep circumferential calcium with well-defined borders. (D) IVUS imaging showing calcium ring that is highly echogenic. IVUS, intravascular ultrasound; OCT, optical coherence tomography.
In a recent study by Wang et al,16 ICA, IVUS and OCT were compared in the assessment of 440 lesions in 440 patients that underwent PCI. Not surprisingly, they found a low sensitivity of ICA for detecting any calcium detected with the intravascular techniques (48.4% against IVUS). However, the specificity of ICA was excellent (98.7%), which along with the fact that angiographically-invisible calcium was thinner, more limited and had a smaller circumferential arc, meant that ICA may be sensitive enough to detect CAC to an extent that it is relevant for planning PCI. In fact, angiographically visible calcium accurately predicted worse stent expansion in these target lesions.
Intracoronary imaging
More detailed characterisation of CAC is possible by using intravascular imaging techniques. Both OCT and IVUS have enough resolution to accurately assess the distribution of coronary calcium in the circumferential and longitudinal axis (figure 1). However, since ultrasound waves do not penetrate calcium, IVUS is unable to reliably assess the thickness of the calcium deposits. On the contrary, the light beam of OCT penetrates calcium in depth without attenuation, allowing a more complete depiction of the calcium deposit. Notwithstanding, the presence of reverberations in IVUS has been suggested as a surrogate marker of thinner calcium16 (online supplemental table 1).
Interestingly, in the study by Wang et al,16 it was shown that OCT missed ~7% (26/364) of the cases in which IVUS detected calcium and underestimated the arc length in ~7% (22/338) of the detected cases. This was presumably due to light attenuation, either owing to the deepness of the deposits or to the presence of a more superficial high-attenuating plaque. Conversely, in only ~1% (4/364) of the cases in which IVUS detected calcification, the arc was underestimated compared with OCT due primarily to an imaging artefact. The conclusions of this study—that OCT may be less sensitive than IVUS—should be received with caution in view of some methodological limitations of this study.17
OCT has excellent accuracy for histologically detected calcium18 19 but some caveats must be considered in the assessment of calcium with OCT. In addition to the light attenuation phenomenon, and the inherent limited axial tissue penetration, necrotic cores might be misinterpreted as calcium deposits, though these are usually demarcated by sharper edge demarcations.20 Fujino et al21 developed an OCT-based scoring system to identify calcified lesions that would benefit from plaque modification prior to PCI. They demonstrated that lesions with calcium deposit with a maximum angle of >180°, maximum thickness of >0.5 mm and length of >5 mm may be at risk of underexpansion.21
One of the major limitations of IVUS are in visualising calcium deposits of small size or when these are located underneath large necrotic cores.22 23 Moreover, dense fibrous tissue also reflects ultrasound and sometimes can generate a hypoechoic shadow, thus mimicking calcium. Contrary to fibrous tissue, calcium tends to produce posterior echo reverberations, which is a highly specific feature.15 Similar to the OCT scoring system, a recently published IVUS-specific scoring system by Zhang et al24 reported that a superficial calcium arc of >270° longer than 5 mm, 360° of superficial calcium, presence of a calcified nodule and vessel diameter of <3.5 mm was associated with stent underexpansion.24 Both techniques are valid for the assessment of the burden of calcium and by using intracoronary imaging with their respective scoring systems in combination with ICA, operators are unlikely to overlook clinically relevant CAC.
CT
Coronary CT angiography has emerged as the preferred first-line modality for the detection of coronary artery disease (CAD) in patients with low–intermediate risk.25–27 Not only is it useful for the detection of CAD, but emerging data supports its use in the planning of PCI given its accuracy for plaque and calcium characterisation.25 CT allows a better depiction of calcium distribution than ICA, but the assessment of thickness is limited due to its low resolution and artefacts. The overall sensitivity of CT to detect CAC has been shown to be higher than that of ICA –80% against IVUS and 78% against histology.28 29 As shown by Okabe et al, and similar to ICA, the sensitivity of CT dropped when the calcium deposits were smaller as measured with IVUS.29 Likewise, in the study by Obaid et al the highest disagreement in measurements of calcified plaque volume between IVUS and CT was seen in plaques with the smallest volumes.28 Therefore, although the overall correlation between calcium quantification by CT and IVUS remains modest,28–30 it seems that CT is also sensitive enough to detect relevant CAC for PCI planning (discussed in the following section).
Guidance of PCI
Imaging characterisation of CAC is of great importance in the preprocedural and periprocedural planning of PCI to choose the appropriate approach, limit the negative acute impact on the outcome of PCI and also ensure long-term durability. Circumferential arc angle, longitudinal extension and axial thickness of the calcium deposits have been shown to determine PCI outcome21 26 31 32 and hence may help to select the lesions that merit the use of adjunctive intracoronary devices. By assessing the various parameters contained within the aforementioned IVUS and OCT scoring systems allows the operator to perform a complete yet simplistic evaluation for the need for plaque modification.21 24
Adjunctive intracoronary devices dedicated for tackling CAC can modify the structure of the calcium deposit by ablating its surface and/or by fracturing its integrity (cracks and discontinuities). Calcium fractures are associated with greater stent expansion and better early and late procedural outcome.33–35 Fujino et al analysed 261 calcified de novo lesions with OCT pre-PCI and post-PCI with only balloon predilatation.35 The authors found that calcifications with large circumferential length plus small minimum axial thickness were more prone to fracture after conventional PCI. Very similar findings were obtained in a smaller study by Maejima et al in which they analysed 37 lesions with OCT before and after PCI with rotational atherectomy (RA).33 Putting all these findings together, it seems that axial calcium thickness is particularly important to determine which lesions require further lesion preparation and thus, OCT may play a prevailing role over ICA and IVUS in this stage of PCI planning.
The non-invasiveness and three-dimensional spatial resolution of CT has potential advantages over ICA in the preprocedural planning prior to PCI. CT coronary angiogram (CTCA) is increasingly becoming a gatekeeper in the evaluation of CAD and to patients entering the catheterisation laboratory. CTCA has been proven beneficial in the assessment of both lumen and vessel structure and in the identification of high-risk plaque features and functional significance of stenosis.26 36–39 In a small study by Sekimoto et al a calcification arc of ≥270° and a high per lesion coronary artery calcium score was found to be predictors of the need for rotablation during PCI.40 However, significant limitations endure with regards to CTCA in calcified lesions. Bloom and streak artefacts may lead to higher rates of false-positive studies, with these artefacts giving the impression of non-calcified plaques with overestimation of plaque burden.26 38 41 This has been demonstrated in a study by Monizzi et al, where calcium volume was overestimated by 60% compared with OCT.42 An increasing number of patients will have a CTCA performed prior to angiography, therefore its potential benefit in procedural planning must be maximised.
A special scenario for CT is the setting of chronic total occlusions (CTO). Fujino et al scrutinised procedural outcomes in 218 CTO lesions undergoing PCI and analysed the predictive power of the J-CTO (multicentre CTO registry of Japan) score calculated on grounds of pre-PCI CT versus J-CTO derived from ICA only.43 The CT-based J-CTO score demonstrated higher predictive value for 30 min wire crossing and procedural success. Among the items of the score, binary calcification by CT (defined as occupying >50% of the vessel cross-sectional area) showed the largest improvement in predictive performance against binary calcification by ICA (CAC visible or not).
Current management strategies for the treatment of calcification
Although previous studies have shown that CAC is present in between 17% and 35% of cases undergoing PCI, its prevalence is likely to increase due to major shift in patient complexity undergoing PCI—largely related to ageing and comorbidities. CAC is usually less responsive to conventional and routine angioplasty techniques employed in non-calcified CAD.44 In this regard, several adjunctive therapies have been developed to aid in the modification of calcified plaques in order to facilitate stent delivery, improve final stent expansion and apposition and ultimately lead to improved clinical outcomes. A comparison of the outcomes of the various adjunctive technologies can be found in online supplemental table 2.
Scoring, cutting and super high-pressure balloons
Scoring balloons AngioSculpt (AngioScore, California, USA), Scoreflex (OrbusNeich Medical, B.V. Hoevelaken, The Netherlands) and cutting balloons such as Flextome and Wolverine (Boston Scientific, Natick, Massachusetts, USA) are modified balloon catheters which create incisions within the lesion to increase balloon expansion during inflation. Cutting balloons have multiple microscopic blades bonded longitudinally on a semi-compliant balloon to create tracts to maximise balloon expansion.45 Preclinical and translational human studies models have shown that cutting balloons create more robust circumscribed lesion preparation with less scar formation and less inflammation than conventional balloons.45 46 This has been corroborated by in vivo IVUS studies which show that acute luminal gain is higher with cutting balloon dilatation, though associated with higher rates of dissection at lower pressure.47 Observational studies have shown that cutting balloon dilatation is associated with higher acute luminal gain and final minimal stent area compared with conventional balloon therapy but, to date, no randomised trials have been performed.48 49 In practice, cutting balloons are often used in conjunction with RA as part of an aggressive lesion preparation protocol but studies have shown conflicting results with this approach compared with conventional balloon therapy.50 51
Scoring balloons are semi-compliant balloons with a nylon helical scoring edge which may enable lesion dilatation while also potentially preventing slippage. Scoring balloons are potentially more deliverable than cutting balloons but there is limited data on their use in calcified arteries.52 OCT has shown their mechanism of action to be intimomedial dissection.53 Use of scoring balloons is predominately restricted to in-stent restenosis, a proportion of which may be due to underexpansion related to fibrocalcific disease.54 55 The role of combining these relatively simple technologies with the more aggressive atherectomy devices remains to be answered. A depiction of the more aggressive atherectomy devices and their respective procedural considerations is described in online supplemental table 3.
Super high-pressure balloons such as OPN NC; (SIS Medical AG, Winterthur, Switzerland), may prove beneficial in this context. With a rated burst pressure of 35 atm, they may prove beneficial for preparation of calcific plaque. Rheude et al randomised patients to predilatation with super high-pressure balloons or scoring balloons prior to DES implantation. The primary endpoint was the stent expansion index assessed by OCT, observing a comparable stent expansion index between the groups. Super high-pressure balloons increased the minimum lumen diameter (2.83±0.34 mm vs 2.65±0.36 mm; p=0.03) and reduced diameter stenosis (11.6±4.8% vs 14.4±5.6%; p=0.02) without a difference in angiographic success.56 Secco et al have also assessed the safety of these balloons, and reported angiographic success in >90% of the cases and an overall MACE rate of 0.9%.57 58 While using OPN balloons may be beneficial in some cases, operators may have concerns regarding the risk perforation and therefore elect to use other techniques such as intravascular lithotripsy.
Ablative atherectomy techniques
The two most widely used ablative atherectomy techniques are rotational and orbital atherectomy, and both will be discussed in detail in the following subsections. The choice to use either technique is largely based on individual experience and availability. In general, RA may be more beneficial in situations when more forward ablation power is required, particularly in cases of abrupt uncrossable lesions. However, orbital atherectomy may be superior when forward-only ablation would be associated with wire biased plaque ablation, which is typical in the case of nodular lesions, at bends or at ostial circumflex locations. By using both anterograde and retrograde ablation, orbital atherectomy may be more effective with less chance of burr entrapment. Finally, by varying the ablation diameter with rotational speed, orbital atherectomy may be more useful in situations where plaque preparation in larger vessels or where ablation of heavily calcified walls is required, situations in which RA is limited by the burr size.
RA
RA (Boston Scientific, Boston, Massachusetts, USA), first introduced by Hansen and colleagues in 1988,59 is an intracoronary device which ablates the calcified plaque with two principal objectives—first to alter plaque rigidity to modify vessel compliance and second to increase luminal area to facilitate delivery of balloons or stents. RA consists of a diamond-encrusted elliptical burr passed over a custom 0.009” guidewire with a 0.014” tip (RotaWire) which rotates at rates of ~150 000 rpm and is advanced with a helical driveshaft. To facilitate forward ablation and non-ablative withdrawal of the rotating burr, only the frontal half of the burr is diamond-encrusted. The equipment is 6 or 7 Fr. compatible depending on the size of burr and can be used through both a radial or femoral route. The addition of the ROTAPRO system (Boston Scientific, Boston, Massachusetts, USA) has streamlined the setup and simplified the controls while maintaining the previous burr and drive shaft technology allowing for a seamless integration into any modern catheterisation laboratory, making it a lesser demanding procedure. Currently, optimal technique recommends sizing the burr: artery ratio as 50%, use of a pecking motion (to avoid macroparticle embolisation), restricting ablations to <20 s and avoiding decelerations >5000 rpm during ablation to avoid complications.60 61 Absolute contraindications for the use of RA are target vessel being a saphenous vein graft, presence of thrombus or prior dissection, though exceptional reports of use in these settings exist.62–66
In retrospective studies conducted in the DES era, RA has been shown to achieve acute procedural success rates of 92.5–100%.67–76 The rate of MACE in this high-risk patient group with high coronary complexity was 6–30.1% and repeat revascularisation was 2.8–35.7% at follow-up (range 6–49 months). These studies were observational, without a control arm and often retrospective so the possibility of selection bias is an important consideration. A recently published prospective European registry by Bouisset et al of 966 patients reported clinical success in 91.6% with low rates of MACE. Factors that were independently associated with MACE at 1 year included female gender, renal disease, ACS at admission, reduced left ventricular systolic function and significant left main disease.76 The first randomised controlled trial of RA was the ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) study. This study involved 240 patients undergoing PCI that were randomised to RA or conventional PCI treatment in moderate to severely calcified lesions. This study found that patients treated with RA had higher rates of acute procedural success (92.5% vs 83.3%, p=0.03) with higher acute luminal gain but also significantly higher rates of late luminal loss at 9 months (0.4 mm2 ± 0.6 vs 0.3 mm2 ± 0.5, p = 0.04). Rates of MACE, TLR and stent thrombosis were equal between both groups.67
The second randomised control trial of RA was the PREPARE-CALC study, which randomised 200 patients with documented myocardial ischaemia and severely calcified lesions to a strategy of modified balloons or RA. This study showed strategy success was more common in the RA group (81% vs 98%; relative risk of failure with an MB-based vs RA-based strategy, 9.5; 95% CI, 2.3 to 39.7; p=0.0001) and RA was not associated with excessive late lumen loss. At 9 months, both strategies were associated with similar clinical outcomes in terms of mortality, MI and target vessel revascularisation rates.77
Periprocedural complications after RA include iatrogenic dissection (1.7–5.9%), coronary perforation (0.5–2.0%), acute closure (0.3–2.0%) and slow-reflow or no-reflow (0.0–2.6%).68–75 Slow reflow is a complication that is particularly pertinent to atherectomy techniques since the plaque abrasion can result in microparticle embolisation, especially with inappropriate technique. By using a slow sanding technique, in addition to the infusion of vasodilator flush cocktail and modern antiplatelet regimes, slow reflow can be significantly reduced, resulting in smaller microparticle embolisation and a reduced degree of microvascular obstruction.78
In theory, RA is thus particularly indicated in lesions with a predominance of superficial calcification since the burr selectively ablates at the luminal surface. This is particularly important where there is wire bias created in calcified arteries with tortuosity. Intravascular imaging using OCT post RA has shown that atherectomy results in a superficial concave groove within both calcified and non-calcified tissue with minimal impact on lumen area.79–82 However, modification of the underlying plaque accounts for the increase in PCI success rates. Another important inherent quality of RA is the formation of a channel (figure 3) to facilitate passing of other adjunctive equipment which are difficult to pass due to size and rigidity, as well as coronary complexity and calcium morphology (ie, sharply protrusive eccentric calcium).
Optical coherence tomography showing the results after sequential use of rotational atherectomy (RA) and intravascular lithotripsy (IVL). (A and B) Two separate cross-sectional frames of a heavily calcified lesion treated with RA. The asterisk shows the cavity formed by the pass of the burr. (C and D) cross-sectional frames after the use of a lithotripsy balloon. The arrow indicates the creation of a deep fracture in the body of the calcium deposit.
Orbital atherectomy
Orbital atherectomy (OA) (Diamondback 360) (Cardiovascular Systems, St. Paul, Minnesota, USA) is an additional atherectomy device that was first introduced in the coronary field in 2013.83 The OA device Diamondback 360 is 6 Fr. compatible and mounted over a dedicated wire—0.0012” ViperWire Advance. It consists of a drive shaft with an eccentrically mounted diamond coated crown which rotates and orbits within the artery, thereby applying a centrifugal force on the calcification. As with RA, the aim of treatment is to modify vessel rigidity, thereby enabling stent delivery. The main difference with RA, however, is that in OA the area of ablation increases with rotational speed, becoming substantially larger than the cross-sectional area of the rotating element. Optimal technique for OA recommends a continuous motion (since the risk of crown entrapment is lower), rapid continuous infusion of the ViperSlide flush and use of retrograde ablation to avoid device entrapment and for a more complete ablation.84
The ORBIT I trial, which recruited 50 patients with severe calcified lesions, had a procedure success of 94% (defined as <20% of residual stenosis after stent placement). Iatrogenic dissection occurred in 12% and the procedural perforation rate was 1.8% but there were no cases of slow reflow.83 Rates of MACE at 6 months were 12.1% which were sustained out to 5 years (21.2%).85 The ORBIT II trial was a larger trial which recruited 443 patients with severely calcified native CAD. In this trial, successful stent delivery was 97.7%, the 1-year MACE rate was 16.4% and the TLR rate was 0.7%.86 At 3-year follow-up, the cumulative rate of 3-year MACE was 23.5%, which included cardiac death (6.7%), MI (11.2%) and target vessel revascularization (TVR) (10.2%). The 3-year target lesion revascularisation rate was 7.8%.87 In a large real-world multicentre registry of 458 cases with severe angiographic calcification, MACE at 30 days was 1.7%, coronary perforation occurred in 0.7%, iatrogenic dissection in 0.7% and no reflow in 0.7%.88 The ECLIPSE trial (ClinicalTrials.gov, identifier NCT03108456) is a randomised controlled superiority trial which is currently recruiting and will compare OA with conventional angioplasty in 2000 patients. The principal primary endpoint is target vessel failure (composite of cardiac death, target vessel-related MI and clinically driven target vessel revascularisation) with an OCT substudy evaluating final minimal stent area.
As with RA, OCT has shown that OA creates gutters within the superficial tissues (both calcified and non- calcified) with limited impact on deeper calcification and lumen area89 90 (figure 4). However, a head-to-head comparison of OA and RA using OCT found that OA created deeper gutters and with potentially more effect on lipid rich lesions. In this study, there was less stent malapposition and a trend towards improved stent expansion.79 Potential advantages over RA include bidirectional treatment of both the proximal and distal shoulders of an atherosclerotic lesion and the ability to treat different vessel diameters and potentially deeper calcification.79 89 90 Despite the differences in design of the ablative element, no comparative studies between OA and RA in terms of forward ablation power and ability to penetrate tight calcific stenoses are available.
Optical coherence tomography showing the results after using orbital atherectomy. (A) Circumferential thick calcium sheet. (B) Corresponding frame after being treated with orbital atherectomy. The arrow shows the concavity formed by the device. (C) Final result after completion of angioplasty and stent implantation.
Laser atherectomy
Laser atherectomy (LA) (Spectranetics, Colorado Springs, Colorado, USA) was first used for peripheral intervention in the 1980s but obtained Food and Drug Administration approval for coronary use in 1992. LA uses xenon-chloride gas as a medium to generate ultraviolet pulses at 308 nm. These pulses exert a photochemical effect where the pulses destroy carbon bonds in vascular material, a photothermal effect where intracellular water is heated and causes cellular destruction and also a photomechanical effect, where bubbles formed at the catheter tip explode and modify obstructive material. The benefit of the 308 nm laser range is that it exhibits less tissue penetration (<30 microM), less heat production with overall less tissue damage than higher laser ranges while maintaining adequate coronary calcification modification. The equipment is compatible with any 0.014” coronary guide wire and is available in four diameters—0.9, 1.4 mm (6 Fr. compatible) and 1.7, 2.0 mm (7 Fr. compatible). The laser catheters are designed with configurations of both concentric and eccentric layering of the fibres for different lesion types. Various repletion rates target different materials, and the 80 Hz is optimal for fibrocalcific lesions.91 92
LA has many potential indications including for organised thrombus, undilatable lesions, in-stent restenosis, chronic total occlusions and acute or chronic stent underexpansion. Several small retrospective registries have evaluated the use of LA in complex undilatable lesions, many of which were due, at least in part, to calcification.92–97 There are many causes of undilatable lesions including fibrotic lesions and calcified lesions and the success of LA in modifying undilatable lesions with primarily severe calcific burden remains under question.92 98 Small cases series have shown OCT images of LA in calcified lesions, which confirm the creation of dissection planes which may ease plaque dilation and delivery of stents.99 A second small case series showed intimal disruption but also with small focal areas of calcium fracture.100
In the LEONARDO study, balloon resistant calcified lesions only were treated with laser with high procedural success (93.7%) and procedural complications were low with no cases of dissection, perforation or slow reflow.101 In the ERBAC trial, 41% of the lesions treated with LA had an element of calcification with a final procedural success of 77.2% and cumulative procedural complications of 4.3%. Rates of coronary dissection were 6.9%, acute occlusion in 1.3% and perforation in 1.3%. The rate of TVR at 360 days was 46.0% (higher than plain old balloon angioplasty (POBA) and death or non-fatal MI was 4.3%.102 In the LAVA study, moderate-to-severe calcification was present in 62% of the cases with high procedural success (88%) with procedural MACE rates of 3.5% and periprocedural complications of 18%. Coronary dissection occurred in 4.3% and coronary perforation in 1.7% . In the AMRO study, 21.8% of lesions were calcified. Procedural success was 96.6%. Coronary occlusion occurred in 5.1% and coronary perforation in 1.7% with a MACE rate (defined as MI, death, coronary artery bypass graft or PCI) of 36.8% at a follow-up of 12 months.103
Complications particular to LA include overheating which may result from cessation of the saline flush or inadvertent contrast administration which could cause vascular injury. Rates of slow reflow are low since the microparticles formed are <10 microM so pass through the coronary microcirculation with subsequent absorption by the reticuloendothelial system. There are no absolute contraindications for LA, with a relative contraindication being intentional or unintentional subintimal passage of the guidewire, such as in antegrade dissection re-entry or retrograde dissection re-entry techniques for CTO PCI.
Intravascular lithotripsy
Intravascular lithotripsy (IVL) (Shockwave Medical, Fremont, California, USA) is a novel technology that delivers acoustic pulse waves circumferentially to disrupt superficial and deep calcification. The system is composed of a balloon dilatable catheter with 0.014” monorail system which is prepared in a conventional manner. It has a dual port hub, one of which is attached to the balloon indeflator and the other to a connector cable, which is coupled to the pulse generator console. Preset pulses are delivered by button activation on the connector cable for 10 s (one pulse/s). The system is compatible with all 0.014” conventional coronary guidewires and is 6 French compatible. An important potential advantage compared with RA and OA, is that IVL can be performed in stenoses located at bifurcations while always maintaining a side-branch wires in place.
Data from the DISRUPT-CAD series of trials, in particular DISRUPT_CAD III,104 which is a prospective multicentre single arm study, have provided early clinical outcome data for the use of IVL. Four hundred and thirty-one patients with highly calcified and complex lesions (mean calcified segment length of 47.9 mm±18.8 mm, calcium angle was 292.5±76.5°, and calcium thickness was 0.96±0.25 mm at the site of maximum calcification) were recruited for this trial. The primary safety endpoint was 30-day freedom from MACE. The primary efficacy outcome was procedural success (stent delivery with a residual stenosis <50% without in-hospital MACE). The use of IVL was associated with procedural success in 92.4% and the primary safety endpoint of 30-day freedom from MACE was 92.2%; which exceeded the prespecified performance study goals (p<0.0001).104 The OCT data from this trial showed multiplane and longitudinal calcium fractures, following the use of IVL in 67.4% of lesions (figures 3 and 5). It also demonstrated that the minimal stent area was 6.5±2.1 mm2 and this was similar irrespective of whether fractures were demonstrated by OCT. While it is clear that further studies are needed, and indeed are ongoing, there is growing evidence to support the safety and efficacy of the use of IVL in severely calcified lesions.104 At 1 year, MACE occurred in 13.8% of the patients (cardiac death: 1.1%, MI: 10.5%, ischaemia-driven target vessel revascularisation: 6.0%) and target lesion failure occurred in 11.9% (ischemia-driven-TLR: 4.3%), both driven by non-Q-wave MI (9.2%). Stent thrombosis occurred in 1.1% of the patients.105
Detailed depiction with optical coherence tomography (OCT) of the effects of intravascular lithotripsy throughout a heavily calcified undilatable lesion. (A) Underexpansion of a 4.0 mm high-pressure balloon in a calcified proximal left anterior descending artery. (B and C) OCT cross-sectional frames showing the presence of thick calcium sheets surrounding the vessel. (D–F) OCT three-dimensional long view reconstruction and cross-sectional frames after the use of lithotripsy showing the cracks formed in the calcium surface fracturing its body creating deep grooves.
Directed use of adjunctive devices in special situations
Bifurcations
Bifurcations are increasingly treated in contemporary PCI practice and can represent up to 15–20% of the PCI activity.106 CAC within bifurcations in a large study of 525 patients was an independent predictor of poor outcomes after RA.107 There is limited data for the use of RA in bifurcations108–110 but with acceptable results. Predictors for side branch compromise in calcified bifurcation PCI include side branch angiographic stenosis and smaller MLD but not main vessel calcium burden.111 Bifurcations were excluded from the ORBIT II trial but observational studies have exhibited acceptable results with this technology.112 113 One study compared the use of RA and OA for bifurcations where OA had shorter procedure time and lower contrast use than RA but there was procedural and clinical equipoise.112 A major disadvantage for both atherectomy devices is that a second wire cannot be placed alongside to protect the side branch. In this regard, LA and IVL hold theoretical advantages over RA and OA since compatibility with conventional wires enables side-branch protection, facilitates recrossing with more controlled lesion preparation. There are limited reports of the use of these technologies in this setting and further trials are required.
Left main stem
PCI to the left main stem (LMS) is increasingly performed, particularly after the results of the EXCEL and NOBLE studies.114 115 Use of RA in LMS PCI was conventionally viewed as a relative contraindication but, with expansion of this procedure, its use has become more routine.62 Use of RA in patients with LMS disease was associated with improved outcomes compared with conventional therapy in a large National registry.116 In a prospective European registry, RA of LM lesions was associated with high clinical success rates with comparable 1-year MACE between RA-LM and non-LM RA group, although target vessel revascularisation was higher in the RA-LM group.117 There are few reports of OA, LA and IVL in LMS PCI (unprotected LMS was an exclusion criterion from the DISRUPT – CAD III). However, a growing body of observational registry data, which includes the use of IVL in unprotected LMS exists and shows that its use in this context is feasible without significant additional associated risks.118 Further randomised studies are of course warranted however to explore this further.
As with bifurcations, the use of LA and IVL hold particular promise for PCI to the LMS, where complexity and jeopardy are both high. In these cases, side branch protection, re-crossing and controlled calcium modification are essential. Furthermore, LMS PCI is often performed in the setting of impaired left ventricular (LV) function where acute complications such as acute vessel closure, perforation and slow reflow can be catastrophic. Technologies that minimise these complications, while providing controlled calcium modification with treatment of deep circumferential calcification are advantageous. While further trials are required, IVL holds particular promise in this regard. When using IVL in the LMS, the operator should consider using shorter applications of IVL to minimise ischaemia given the large territory involved, Finally, it is important to note that use of LV support devices, particularly in cases with reduced left ventricular ejection fraction may be of particular benefit by permitting haemodynamic protection and enabling more capacity for vessel preparation.119–121
In-stent restenosis
In-stent restenosis (ISR) occurs in 10% of the patients with second-generation DES and remains an important cause of morbidity.122 Our understanding of the mechanisms of ISR have expanded significantly with the advent of high-resolution intracoronary imaging.123 OCT holds significant potential in delineating the mechanisms of ISR and the nature of the ISR—in particular differentiating between neointimal hyperplasia and neoatherosclerosis and also the cause of the stent failure, particularly underexpansion due to deep circumferential calcification.124 In this clinical context, it is important to differentiate between neoatherosclerosis with fibrocalcific plaque versus stent underexpansion due to calcific nodules or deep circumferential calcium. Stent underexpansion is more frequent, however neoatherosclerosis with calcific plaque perhaps may be more amenable to treatment and potentially may be safer, although the dilatation of these lesions will be restricted by the underlying stent expansion limit. It stands to reason that neoatherosclerosis with evidence of fibrocalcific disease may be more amenable to atherectomy treatments that treat superficial calcification125 and, that if the mechanism of stent failure was due to chronic underexpansion from circumferential calcification, that ability to modify this deep calcification, may improve the long-term success of the PCI.
RA was found to be beneficial in treating ISR when the mechanism was due to neointimal hyperplasia on IVUS in the ROSTER trial, where it was associated with lower rates of TVR, higher stent expansion (10% vs 31%, p<0.001) and target lesion revascularisation (45% vs 32%, p=0.042) in 200 patients with IVUS-guided PCI compared with balloon therapies.126 In the ARTIST trial, however, use of RA was less effective than balloon therapy with higher rates of residual underexpansion and restenosis. However, this trial also included patients with underexpanded stents on IVUS,127 which may have been due to deep calcification which is potentially less amenable to the inherent properties of RA.
LA has been shown to improve acute luminal gain compared with conventional balloon technologies in the treatment of ISR in one IVUS study.128 In this study, there was a trend to reduce TLR at 6 months (21 vs 38%; p=0.083). Concerns about thermal injury of the metallic struts have not been borne out in a porcine stent model or in bench tests.129 LA may be particularly beneficial in cases of fibrotic neointimal hyperplasia as opposed to neoatherosclerosis.
There is limited data on the use of OA and IVL in ISR but this may hold particular potential for cases of severe underexpansion due to deep circumferential calcification. Several reports on successful treatment of severe chronic stent underexpansion, refractory to very high-pressure balloon dilatation, suggests that IVL constitutes a valuable alternative in this challenging scenario.130–132 IVL thus offers a novel alternative in this clinical situation with previously limited treatment options, and this merits further evaluation.
Chronic total occlusion
Severe calcification is present in 58% of the CTOs and is associated with higher J-CTO scores, more retrograde approaches, longer procedural time, use of higher contrast volumes and lower procedural success.133 Balloon uncrossable lesions occur in 6.4–9% of the CTO cases and severe calcification is invariably present.134 RA has been used with success in CTO cases with significant burden of calcification with similar overall outcomes to non-CTOs, and often with use of smaller burr: artery ratios.135–137 RA is particularly useful in balloon uncrossable or undilatable lesions where it achieved 97% success and was also used with success in dissection re-entry cases.138 LA has the potential to weaken bonds in the cap of the CTO and there are additional potential antithrombotic and antiplatelet benefits of LA which may reduce the risk of periprocedural complications.139 140 The acute procedural success rate of LA in CTO is reported to be as high as 90% but these data originate from older studies which may not accord with contemporary PCI.141 There is limited data of the use of OA and IVL in CTOs currently. A major advantage of IVL is its inherent compatibility with all coronary wires but a current limitation is the bulkiness of the device which may prevent deliverability, particularly in uncrossable lesions.
Aorto-ostial lesions
Aorto-ostial lesions are associated with higher rates of acute vessel recoil, higher rates of in-stent restenosis and TVR.142 143 Calcium modification devices may improve procedural success and long-term clinical outcomes. RA has the benefit of improving crossability while modifying vessel compliance and facilitating stent delivery.144 145 One study of the use of RA in RCA ostial lesions (n=119) was associated with high procedural success (97.5%) and TVR at 2 years of 16% and a smaller retrospective study showed improvement in luminal diameter compared with conventional PCI.146 A further retrospective study by Quillot et al demonstrated similar high procedural success rates in patients with aorto-ostial disease, however 30-day (11.3% vs 4.8%, p=0.04), and 24-month MACE rates were significantly higher in the aorto-ostial group (43.8% vs 31.8%, p=0.04).147 OA may be of use when the lesion can be crossed and may enable more complete lesion ablation, particularly with the use of retrograde ablation. Lesions that cannot be crossed in a non-spinning state can be crossed with application of the OA and, in these situations, the guide should be co-axial and great care should be applied at the aorto-ostial interface. Aorto-ostial lesions were excluded from the ORBIT II trial but preliminary results suggest OA can be used with high procedural success.137 148 There is limited data on the use of LA and IVL in aorto-ostial lesions. Due to over-hanging of aortic calcification, aorto-ostial lesions often exhibit deep calcification. IVL can enable controlled modification of this deep calcification, particularly in cases where the lumen area is large and a 1:1 balloon to artery ratio can be applied.
Conclusions
CAC is an increasingly important challenge for the cardiologist. Recent advances in both intracoronary imaging and adjunctive technologies provide a unique opportunity for a tailored and dynamic approach to its percutaneous treatment. We feel that intracoronary imaging could play an increasingly important role in the evaluation of CAC in order to guide subsequent treatment. In particular, OCT enables clarification of the arc and depth of calcium, which together may guide atherectomy strategies and, in addition, the efficacy of the ablation prior to final stenting. As such, prospective studies evaluating OCT-guided protocolised atherectomy treatments against current standard of care should be conducted.
Furthermore, there has been an expansion of tools to manage CAC in the interventional cardiologist’s armoury and rather than being viewed as conflicting, they should be considered in concert (figure 6). In particular, the relative advantages of the different technologies in different clinical situations, (eg, bifurcations, LMS, ISR) should be exploited maximally. Notwithstanding, data on the efficacy of the relative treatments is scant and we should strive to conduct high quality prospective randomised controlled trials. The ability to show superiority for clinical outcomes in this realm can be challenging without adequately powered studies but proof of concept studies with intracoronary imaging and coronary physiology studies are crucial to then set the scene for larger scale clinical studies.
Coronary calcification management algorithm. CAC, coronary artery calcification; IVL, intravascular lithotripsy; LA, laser atherectomy; OA, orbital atherectomy; OCT, optical coherence tomography; NC, non-compliant; RA, rotational atherectomy.
As the appropriateness criteria for PCI expands, it is increasingly incumbent on the interventional cardiologist to strive for an excellent stent result, regardless of the challenges. CAC has traditionally been an important obstacle to achieving high success in PCI. With the combination of intra-coronary imaging and an expansion in adjunctive technologies, we are at reaching a new dawn in overcoming this old and familiar foe.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors JH and JE: conception and critical review. NG and ES: media and critical review. BH, NP, FM and JY: research, design and writing.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests NG reports speaker and consultancy fees from Abbott, Boston Scientific, Phillips and Shockwave. JH declares stocks in Shockwave Medical (SWAV).
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.