Article Text
Abstract
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia encountered in clinical practice. One of its most devastating complications is the development of thromboembolism leading to fatal or disabling stroke. Oral anticoagulation (OAC, warfarin) is the standard treatment for stroke prevention in patients with AF with an increased stroke risk. However, there are several obstacles to long-term OAC therapy, including the risk of serious bleeding, several drug–drug interactions and the need for frequent blood testing. Although newer oral anticoagulants have been developed, these drugs also face issues of major bleeding and non-compliance. Therefore, alternative treatment options for stroke prevention in patients with AF with a high stroke risk are needed. Percutaneous left atrial appendage (LAA) occlusion is an evolving therapy, which should be taken into consideration in those patients with non-valvular AF with a high stroke risk and contraindications for OAC. This article aims to discuss the rationale for LAA closure, the available LAA occlusion devices and their clinical evidence until now. Moreover, we discuss the importance of proper patient selection, the role of various imaging techniques and the need for a more tailored postprocedural antithrombotic therapy.
- Allied Specialities
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/
Statistics from Altmetric.com
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, affecting 3–5% of the population aged 65–75 years, and increasing to >8% of those older than 80 years.1–4 It is associated with substantial mortality and morbidity, particularly due to fatal or disabling stroke. The risk of ischaemic stroke in patients with non-valvular AF (NVAF) is 3–5%/year, which is a fivefold increase compared with the unaffected population (figure 1). Overall, AF accounts for 15–20% of strokes in the general population and for up to 30% in patients over the age of 80 years.5–9
The CHA(2)DS2-(VASc) stroke risk and HAS-BLED bleeding risk index are calculated by totalling the scores for each risk factor present.68–71 The lower graph shows the expected stroke rate /100 patient (pt)-years, stratified by CHADS2 score in patients with AF not taking warfarin. Gage (2001): adjusted stroke rates/100 pt-years, assuming that aspirin was not taken68; Gage (2004): stroke rates/100 pt-years of aspirin69; Olesen (2011): event rates of hospital admission and death due to thromboembolism in patients with AF not taking warfarin.70 AF, atrial fibrillation; CHF, congestive heart failure; TIA, transient ischaemic attack; INR, international normalised ratio.
For prevention of this complication, oral anticoagulation (OAC) is the standard treatment in patients with AF with a CHA(2)DS2-(VASc) stroke risk score ≥1.10 This anticoagulant therapy has been proven to effectively prevent thromboembolic strokes, but the increased risk of serious bleeding prevents many patients from taking this therapy.11 ,12 Therefore, alternative treatment options for stroke prevention—without increasing the risk of bleeding—in patients with AF with increased stroke risk are needed.
Percutaneous left atrial appendage (LAA) occlusion is an evolving therapy, which should be taken into consideration in those patients with AF with a high stroke risk and contraindications for OAC.10 ,13 As a relatively new invasive procedure, it still needs to be demonstrated that its efficacy in preventing stroke outweighs the possible complications it may cause. This review aims to discuss the rationale for LAA closure, the available LAA occlusion devices and their clinical evidence until now. Moreover, we discuss the importance of proper patient selection, the role of various imaging techniques and the need for a more tailored postprocedural antithrombotic therapy.
Treatment options for stroke prevention
Multiple, large randomised controlled trials (RCTs) have clearly established the efficacy of OAC therapy in lowering the risk of stroke and death in patients with AF with a high stroke risk.10 However, there are several obstacles to long-term OAC therapy. The biggest risk is major bleeding, which has an incidence of 2–4%/year14–16 and can be even higher if predisposing factors are present. In addition, these drugs have a small therapeutic window, several food and drug interactions and require frequent blood testing, which makes this therapy inconvenient to many patients. As a consequence, OACs are currently not prescribed and/or rigorously taken in up to 50% of patients with AF who are at high risk for thromboembolic events.11 There has been a large hope that the new oral anticoagulants (NOACs) would overcome these disadvantages; however, these drugs still face issues of major bleeding and non-compliance (table 1) and have been reported to have side effects like gastrointestinal intolerance.14–16
Comparison of some clinical data from the PROTECT AF versus new oral anticoagulant trials *
An appealing way to avoid the need for OAC therapy could be complete eradication of AF. However, so far, none of the strategies trying to control and/or eradicate AF—either by medical rhythm control or radiofrequency catheter ablation (RFA)—have been proven to eliminate the indication for long-term OAC therapy. Although a recent study suggests that patients who undergo RFA may have a lower risk of stroke than patients with AF who do not undergo RFA,17 the guidelines (so far) do not recommend discontinuation of OAC after AF ablation.10 ,18
Rationale for LAA occlusion
The rationale behind LAA occlusion has been derived from anatomic and echocardiographic findings identifying the LAA as the primary site of thrombus formation in patients with NVAF. In a review of 23 studies in which the LAA was examined by autopsy, direct intraoperative inspection or transoesophageal echocardiography (TEE), an intracardiac thrombus, was identified in 10–15% of cases of valvular AF and NVAF—however, the anticoagulation status of these patients was not reported. Sixty per cent of atrial thrombi in valvular AF were found in the LAA, whereas in NVAF >90% of thrombi were located in the LAA.19 Thus, with the LAA found to be the dominant source of thrombus in patients with NVAF, LAA closure (or exclusion) provides an appealing option for stroke prevention.19–21 Undoubtedly, in patients with AF with comorbidities, embolic strokes can also develop from non-cardiac origins such as atheromatous plaque in the thoracic aorta or carotid arteries.20 ,22 Nevertheless, for patients with NVAF with contraindication(s) to OAC, the rationale for LAA occlusion is that this intervention reduces the risk of AF-induced stroke in such a range that it outweighs the possible risk on procedural complications. Whether this hypothesis applies to the different closure devices needs to be proven in future clinical trials. Consequently, the European Society of Cardiology (ESC) guidelines for the management of AF (2012) recommend that LAA closure may be considered in patients with a high stroke risk and contraindications for long-term OAC administration (Class IIb, level of evidence B).10
Patient selection
Percutaneous LAA occlusion offers an alternative to physicians who are facing a complicated risk–benefit analysis in patients with NVAF who should receive OAC therapy based on a high stroke risk score, but who also have a high bleeding risk. Theoretically, all patients with NVAF with increased stroke risk and contraindication(s) to OAC are possible candidates for LAA closure—patients who have no contraindication for OAC therapy should have LAA occlusion only in exceptional cases.13 ,23–25
In clinical practice, patients with NVAF with a high stroke (CHADS2) and bleeding (HAS-BLED) risk score are considered the most suitable candidates for this procedure. Recent data suggest that the higher the stroke risk of the individual patient, the larger the ‘net clinical benefit’ of LAA closure in comparison to OAC therapy.26 However, patients with a high risk for thromboembolism—for example, left ventricular (LV) dysfunction or prior stroke—have also been reported to be at a higher risk of thrombus formation in the LA cavity (and not in the LAA).27 Therefore, an assessment that considers the combined risk of stroke, thromboembolism, bleeding and other adverse events may be the best way to select patients most suitable for LAA closure (boxes 1 and 2).
Box 1 Possible indications for percutaneous LAA closure
-
Patients with AF at high stroke risk with
-
High risk (or recurrence) of bleeding under (N)OAC due to
-
Uncontrolled, severe hypertension
-
Coagulopathies—low platelet counts, myelodysplastic syndrome (MDS)
-
Inherited bleeding disorder—Von Willebrand disease, haemophilia
-
Severe hepatic or renal dysfunction—eg, alcoholic liver cirrhosis
-
Vascular disease or malformations— eg, intestinal angiodysplasia, Osler-Weber-Rendu previous intracerebral haemorrhage, cerebral microbleeds (∼amyloid angiopathy), retinal vasculopathy
-
Insufficiently treatable GI disease with bleeding—eg, neoplastic disease, intestinal angiodysplasia
-
Recurrent nephrolithiasis
-
High probability of frequent and/or severe traumas—eg, epilepsy, in the elderly
-
-
Ischaemic stroke despite well-controlled OAC therapy
-
High probability of therapeutic non-compliance to (N)OAC
-
Intolerance to (N)OAC drugs—GI intolerance, severe liver/kidney dysfunction, drug interactions
-
Other contraindications for (N)OAC
AF, atrial fibrillation; GI, gastrointestinal; LAA, left atrial appendage; (N)OAC, (new) oral anticoagulants.
Box 2 Contraindications for percutaneous LAA closure
-
Low risk for stroke CHA(2)DS2-(VASc)=0
-
Valvular heart disease (eg, mitral stenosis)
-
Other indications for long-term or lifelong OAC—mechanical prosthetic valve, pulmonary embolism and deep vein thrombosis, thrombi in the left atrium or ventricle
-
Contraindications for transseptal catheterisation—left atrial thrombus or tumour, active infection, uncooperative patient, (presence of ASD/PFO closure device)
ASD, atrial septal defect; LAA, left atrial appendage; OAC, oral anticoagulants; PFO, patent foramen ovale.
Besides clinically identifying the most suitable patients with NVAF for LAA occlusion, it is also important to assess the technical feasibility of percutaneous LAA closure in every single patient by means of a preprocedural TEE and/or CT imaging. Some specific aspects of the LAA should be assessed, as this may influence device/size selection and implantation success. Importantly, a thrombus in the LAA is regarded as a contraindication for device implantation—in these cases, patients should take preprocedural OAC therapy until the thrombus resolves.13 ,28
LAA anatomy
The LAA has a highly variable anatomical structure and may be difficult to describe—however, it is important to correctly evaluate the LAA anatomy in order to optimise procedural success. The best way to determine the configuration and orientation of the LAA lobes is by CT and/or angiography; the LAA orifice and neck can also readily be examined by TEE.29
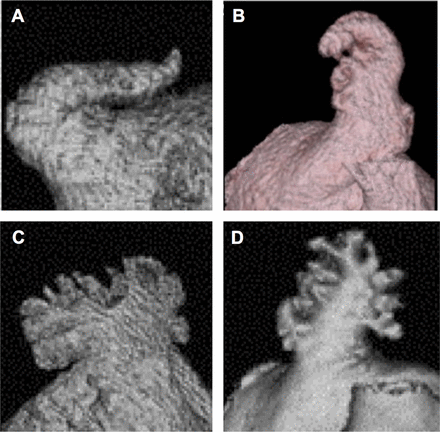
Most often, the LAAs are classified into four morphological groups: (1) chicken wing: an LAA with an obvious bend in the proximal part of the dominant lobe; (2) windsock: an LAA with a main lobe of sufficient length (>4 cm) as the primary structure; (3) cauliflower: an LAA that has limited overall length (<4 cm) without any forked lobes; (4) cactus: a dominant central lobe with secondary lobes extending from the central lobe.30 The frequency of the different morphologies varies in the different studies, which may be either an expression of the difficulty to classify the individual morphology or a consequence of different patient populations and/or definitions in the studies (figure 2).30 ,31
General morphology classification of left atrial appendage (LAA) as determined by cardiac CT. LAA can be classified into four types: (A) chicken wing type—with a short neck and an obvious bend, (B) windsock type, (C) cauliflower type and (D) cactus type. Images reproduced with permission from Wang et al.30
Recent data suggest that the ‘chicken wing’ morphology may be associated with a lower stroke risk32—in some cases, this morphology is a procedural challenge and a modified implantation technique may be preferred (figure 3).33 ,34 In contrast, the ‘cauliflower’ morphology as well as a smaller LAA orifice, larger neck dimension and extensive LAA trabeculation are associated with a higher stroke risk, as these factors potentially cause stasis and consequently thrombus formation in the LAA.35–37
(A–D) Preprocedural transoesophageal echocardiography (TEE) imaging—left atrial appendage (LAA) anatomy and dimensions should be studied with the transducer array rotated through 0°, 45°, 90° and 135°. (A) Shows in which manner the LAA ostium, LAA neck width (orifice width or ‘landing zone’) and LAA depth should be measured. The LAA neck width is typically measured in a plane from the LCx coronary artery to a point 10 mm distal to the limbus (white arrow) of the left superior pulmonary vein (LSPV). In this case, we measured a neck width of 21–25 mm—which led to the choice of a 28 mm Amplatzer cardiac plug (ACP) device (because of oversizing with 3 to 5 mm). The risk of undersizing is device embolisation; the risk of oversizing is compression on the LCx and/or LSPV, as well as LAA perforation and device embolisation. (E–H) Use of ICE to guide implantation of a LAA closure device. ICE imaging is optimal with the ICE probe in (E) the left pulmonary artery as well as (G) with the transducer at the ostium of the coronary sinus. The ICE images were used to guide (F) the delivery and (H) proper deployment of the ACP device inside the LAA. DC, delivery cable; *disk of the ACP device; # lobe of the ACP device.
From an interventional perspective, understanding the different configurations of the LAA ostium and neck is vital, as the occluder is anchored at the neck and must cover the ostium. A recent paper described three different morphologies of the LAA ostium and neck: (1) horn shaped: LAA ostium wider than the neck, (2) parallel tube: an ostium and neck that are similar in dimension and (3) and angel wing: a neck with larger dimensions than the LAA ostium. These various LAA configurations can influence device/size selection and implantation success. The risk of device dislodgement is believed to be highest in patients with a horn-shaped configuration of the LAA. In these patients, choosing a device large enough to cover the ostium and yet maintain the optimal degree of oversizing in the ‘landing zone’ to secure anchorage can be a challenge. Moreover, other aspects such as the amount of trabeculation of the ‘landing zone’ and the depth of the LAA (especially important if using the WATCHMAN device) need to be assessed to ensure that the optimal device is chosen.29
Imaging of the LAA
TEE is an essential tool at all stages of a percutaneous LAA occlusion procedure: (1) preprocedural TEE is used to screen suitable candidates and to define LAA morphology and dimensions; (2) periprocedural TEE has a major role in guiding delivery and deployment of the device and for assessing procedural complications; and (3) postprocedural TEE is important in the surveillance and monitoring of long-term outcome.38–40
As stated above, an extensive preprocedural TEE examination should be performed in order to fully explore the LAA anatomy and to exclude a thrombus in the LA(A). Important aspects to assess are the shape and size of the ostium, the width of the ‘landing zone’ (ie, area within the LAA where the device will be positioned), the length of the LAA and—if possible—the number, shape and location of the lobes (figure 3).38 In case of a large LAA neck width (>26 mm) or complicated LAA anatomy, additional preprocedural imaging with CT/MRI should be considered. This is because all devices have a certain upper limit in size (see figures 4 and 5), and a good preprocedural preparation can prevent the need for interrupting LAA closure procedures after induction of general anaesthesia.30 ,41–43 Finally, it is important to mention that the LAA size is dependent on the LA pressure (‘loading status’) as well as on the presence of sinus rhythm or AF. Therefore, some operators suggest measuring LAA dimensions during the procedure after establishing an LA pressure of more than 10 mm Hg by saline infusion.
PLAATO device. (A) The PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) system was the first device specifically developed for left atrial appendage (LAA) occlusion. It consisted of a self-expanding nitinol cage with three anchors on each strut and was covered with a non-thrombogenic PTFE membrane. The anchoring barbs provided the stability; the PTFE membrane prevented mobilisation of thrombi from the LAA and promoted healing. The device diameter ranged between 15 and 32 mm and was normally selected 20–40% larger than the diameter of the LAA ostium. The device is no longer available for clinical use after withdrawal from the market in 2006. (B) Shows—from left to right—a fully collapsed, partially expanded and fully expanded device advanced through a 12-Fr transseptal delivery sheath. (C, D) Illustrate a fluoroscopic right anterior oblique view in a patient, exhibiting the deployed PLAATO device (C) and in the setting of an LA angiogram (D) depicting proper LAA occlusion. Images reproduced with permission from Aryana et al.47
WATCHMAN device. (A–C) Show the delivery (A), deployment (B), and release (C) of the WATCHMAN device through a 12-Fr transseptal delivery sheath. (D) Shows a close-up view of the WATCHMAN device—consisting of a self-expanding nitinol frame covered with a porous filtering PET membrane. The stability of the device is secured by fixation barbs located circumferentially; the PET membrane acts as a filter preventing the outflow of the thrombi and promotes endothelialisation. The device is available in five different sizes ranging from 21 to 33 mm, and is normally selected 10–20% larger than the left atrial appendage (LAA) diameter to ensure stable device positioning. The device can be recaptured and withdrawn in case of suboptimal fixation. The WATCMAN device received CE-mark approval in 2005 and is currently used in clinical practice. (E) Shows a transoesophageal echocardiography image of an occluded LAA following deployment of a WATCHMAN device—the delivery cable is still connected to the device. (F) Shows a cine image of an LA angiogram demonstrating a WATCHMAN device properly deployed inside the LAA (black arrow). TSS, transseptal sheath. Images reproduced with permission from Aryana et al.47
Device implantation
Percutaneous LAA occlusion is usually performed under general anaesthesia and with TEE and fluoroscopic guidance. Antibiotic prophylaxis is administered prior to the procedure. Vascular access through a femoral vein is obtained and the delivery system is introduced by transseptal puncture using a standard transseptal needle and sheath. In order to have good alignment with the axis of the LAA, the puncture site is preferably inferior and posterior in the fossa. Additionally, different curves of sheaths are available to access the LAA. After the puncture, a bolus of unfractionated heparin should be given to achieve an activated clotting time (ACT) >250 s. TEE (or intracardiac echocardiography (ICE)) and contrast angiography of the LAA (right anterior oblique 30°/cranial 30°) are used to measure the LAA dimensions (ostium, neck width, depth)—based on these measurements, the size of the device is chosen. The optimal C-arm angulation for implantation can vary widely from patient to patient. The positioning of the device in the LAA cavity is ensured by TEE/ICE and fluoroscopy (figure 3). The device is deployed by withdrawing the sheath over the device (to reduce the risk of perforation). Once in position, the device stability is confirmed by a ‘tug test’, and complete sealing is verified by colour Doppler imaging. Finally, the device is released from the delivery cable and possible complications such as pericardial effusion are ruled out. The individual steps may vary with the different devices.13 ,44–46
Available devices
The percutaneous LAA transcatheter occlusion (PLAATO) device was the first to be successfully implanted in humans; however, this device is no longer available after withdrawal from the market in 2006. Until now, the WATCHMAN and ACP device have been most widely used in clinical practice. Very recently, the Coherex WaveCrest device also obtained CE Mark approval for percutaneous LAA occlusion.47 ,48
A detailed description of these different LAA occluders can be found in figures 4–⇓7. References to step-by-step descriptions of a percutaneous LAA closure procedure with a WATCHMAN and ACP device can be found in online supplementary file 1.49–51 Surgical/thoracoscopic LA appendectomy and the epicardial LARIAT suture delivery device are described elsewhere.52–54
(A-B) the ACP device consists of a lobe and a disk connected by a short, flexible waist. Both the lobe and disk are constructed from a nitinol mesh covered with a polyester patch. The lobe is implanted within the neck of the LAA (the so-called ‘landing zone’), and achieves device stabilization and retention by means of a number of stabilization wires. The delivery system is 9-13Fr depending on the size of the device. The lobe size ranges from 16-30 mm and the disk from 20–36 mm; the size of the lobe should be chosen 3 to 5 mm larger than the diameter of the ‘landing zone’. The ACP device is not designed to fill the LAA but to seal its ostium by means of the larger disk. As such, the ACP device could be a better choice when challenged with a more complex anatomy of the distal LAA or a proximal LAA lobe. The ACP device received a CE mark in 2008 and is currently used in clinical practice. (C-D) show the implantation of an ACP device in the regular way (C), or using the ‘sandwich technique’ when confronted with a chicken wing LAA with a short neck (D). (E) a cine image of a LA angiogram performed through a transseptal catheter following deployment of an ACP device (white arrow) inside the LAA - when properly positioned, the lobe has a typical ‘tire’ morphology with slight compression on the sides. (F) a TEE image of a properly deployed ACP device, showing absence of peri-device leaks, good alignment of the disk with the LA cavity, and absence of compression on the left upper pulmonary vein. Some images were reproduced with permission from Aryana et al. (2012).42
(A-B) the Coherex WaveCrest LAA occlusion system is the latest development in LAA occlusion devices. It consists of a nitinol frame with retractable coils and anchors to enable optimal device positioning. The device consists of a multi-composite membrane including a PTFE membrane on the LA side of the device, and a foam substrate on the LA-opposing surface to minimize residual leaks. Very recently, the WAVECREST I clinical trial was completed - CE Mark approval was obtained in September 2013. (C) a cine image showing contrast injection distally of the device (into the LAA) in order to assess complete LAA closure. (D) a TTE image confirming the device is well-seated and without leaks. Some images were presented by Franzen O. on EuroPCR 2013.43
Postprocedural considerations
Many centres perform a chest X-ray and TTE before discharge in order to rule out device embolisation and pericardial effusion. After 45 days, a control TEE is suggested to verify complete sealing of the LAA and the absence of a thrombus.
Various antithrombotic regimens have been used after LAA closure. In the PROTECT AF trial, OAC was given for 45 days and patients discontinued OAC if the 45-day TEE control showed either complete closure of the LAA or if the jet width of the residual peridevice flow was <5 mm. After stopping OAC therapy, patients were given dual antiplatelet therapy (DAPT) with acetylsalicylic acid (ASA) and clopidogrel until completion of the 6-month TEE control, after which ASA monotherapy was continued indefinitely. In 14% of patients, OAC was continued beyond 45 days, and in 8% of patients, OAC was continued beyond 6 months because of incomplete LAA closure or device thrombosis.55
A more recent study—the ASAP registry—of 150 patients receiving the WATCHMAN occluder reports that WATCHMAN implantation can be safely performed without OAC transition and that DAPT prescribed for 6 months followed by ASA alone may be an adequate antithrombotic regimen.56 With the use of the ACP device, OAC has been avoided and DAPT has been prescribed for variable durations, from 1 to 6 months after implantation, followed by ASA alone.
A literature search, however, shows that the incidence of device thrombosis is not insignificant, with rates ranging from 4% to 17%.56–58 The incidence of thrombus formation is more frequent in the first few weeks/months after implantation and significantly declines with complete endothelisation of the occluder surface. In cases of device thrombosis, subcutaneous heparin is typically given for 2 weeks followed by TEE, and DAPT therapy is extended for a longer period.
Interestingly, a recent study involving 34 ACP device implantations reported that the risk for thrombus formation on the device was increased in patients with a high platelet count, high stroke risk score and low LV ejection fraction.57 These findings underline the importance of close follow-up of such patients and the need for a large prospective trial to address the optimal antithrombotic regime and duration of therapy in these high-risk patients.
In clinical practice today, DAPT is prescribed for the first 45 days after LAA closure and clopidogrel can be stopped if major peridevice leaks and device thrombosis are ruled out at the 45-day TEE control. After 6 months, ASA treatment may or may not be stopped completely if no other indication for antiplatelet therapy exists. In patients with a very high bleeding risk, it may be considered to give only one antiplatelet drug from right after the procedure. Only in very exceptional cases, an LAA closure device may be implanted in patients who cannot take any antithrombotic or anticoagulant therapy. In patients with a stroke under anticoagulant therapy, we believe it should be considered to continue low-dose NOAC therapy after LAA closure. Clearly, more studies are needed to determine the optimal postprocedural antithrombotic strategy.
It is noteworthy that it was recently reported that also after percutaneous LAA exclusion with the Lariat snare device—in which no intracavitary device is left behind—thrombus formation at the LAA ostium can occur.59 Therefore, postprocedural antiplatelet therapy is also indicated for this device.
Procedural safety and short-term outcome
Percutaneous LAA occlusion is a procedure for stroke ‘prevention’ in patients with AF, and therefore procedural safety is paramount.
In the PROTECT AF trial (2009)—comparing the WATCHMAN device (n=463) to warfarin (n=244) in patients with AF with increased stroke risk—the primary safety endpoint (composite of pericardial effusion, device embolisation, major bleeding and procedure-related stroke) was increased in the WATCHMAN group (7.4 events/100 patient-years) when compared with the control OAC group (4.4 events/100 patient-years). Most of the events occurred within the first 7 days after device implantation—about 50% were pericardial effusions requiring drainage.55
More recent data, however, from the CAP registry (n=566, WATCHMAN),60 the PREVAIL study (n=269, WATCHMAN)61 and a large multicentre study involving the ACP device (n=969)62 show improved procedural safety—complication rates within 7 days were 4.1%, 4.4% and 4.1%, respectively, as compared with >7% in the initial PROTECT AF trial (2009). These lower rates of periprocedural major adverse events can be ascribed to a better understanding of the procedure and an operator learning curve effect.
A list of possible complications can be found in table 2. A detailed overview of procedural safety and short-term outcome data from the different LAA closure devices is given in table 3.
Possible complications of percutaneous LAA closure
Procedural safety and short-term outcome of percutaneous LAA occlusion
Intermediate/long-term clinical outcome
Most of the studies published until now are non-randomised cohort studies or prospectively collected, retrospectively analysed multicentre registries, which demonstrate that annualised stroke rates after percutaneous LAA closure are favourable when compared with expected stroke rates as predicted by a CHADS2 score (table 4).
Intermediate/long-term outcome data following percutaneous LAA occlusion
The only prospective, randomised trial so far published is the PROTECT AF trial in which 707 patients with NVAF were randomly assigned in a 2:1 ratio to either percutaneous LAA occlusion with the WATCHMAN device or to warfarin therapy. The study was designed to assess the non-inferiority of the device against chronic OAC therapy. Patients with paroxysmal, persistent or permanent NVAF were eligible for enrolment if they had a CHADS2 risk score ≥1. The trial confirmed the non-inferiority of WATCHMAN LAA occlusion compared with OAC therapy regarding the primary efficacy endpoint—a composite of stroke, systemic embolism and cardiovascular death (RR=0.62, 95% CI 0.35 to 1.25). The probability of non-inferiority of the intervention was >99.9%.55
At the Heart Rhythm Society's 34th Annual Scientific Sessions (2013), 4-year follow-up results from the PROTECT AF trial were presented. The primary efficacy endpoint occurred in 2.3% of the device group vs 3.8% of the warfarin group (RR=0.60, 95% CI 0.41 to 1.05), demonstrating a 40% relative risk reduction in primary efficacy in the WATCHMAN group. Cardiovascular death occurred in 1.0% of the device group vs 2.4% of the warfarin group (RR=0.40, 95% CI 0.21 to 0.72, p<0.05). The rate of haemorrhagic stroke was 0.2% in the device group vs 1% in the warfarin group (RR=0.18, 95% CI 0.04 to 0.60, p<0.05). In conclusion, these long-term follow-up data seem to provide additional support for LAA closure as a potential alternative to OAC therapy in patients with NVAF.63
Finally, we also looked at the prevalence and impact of residual peridevice leaks after LAA closure. Peridevice leaks are typically evaluated by colour Doppler (TEE) and classified as: (1) severe—multiple jets or free flow; (2) major—jet width >3 mm; (3) moderate—jet width of 1–3 mm and (4) minor—jet width <1 mm. Major residual leaks have been reported in as many as 62%, 32% and 10% of the patients after PLAATO, WATCHMAN and ACP device implantation, respectively. The lower rate of residual leak observed after ACP implantation may be related to the double-disk structure of the ACP, which may contribute to a better sealing of the LAA orifice. Most importantly, however, the presence of residual peridevice leaks has never been associated with cardioembolic events in any of these studies.64–66 Typically, when detecting major residual peridevice leaks, OAC therapy is continued or restarted for several weeks/months until the next control TEE in the hope of a complete closure or jet width <3 mm. Still, more research about this issue is clearly warranted.
Discussion
Since the first percutaneous LAA closure in 2002, a rapidly growing number of studies have shown that this strategy can be a safe and efficacious therapy in the management of patients with NVAF for whom OAC is indicated but who are at a high risk of bleeding. As a result, the WATCHMAN device received substantial endorsement from a Food and Drug Administration (FDA) advisory panel in December 2013—a definitive FDA approval is expected later this year.
Although the only RCTs available until now (PROTECT AF, PREVAIL) included only patients who were eligible for OAC therapy,55 ,61 we believe that the current data only justify the use of this therapeutic option in patients with contraindications to OAC therapy. Patients with NVAF who have no contraindication(s) for OAC should have LAA occlusion only in exceptional cases. Accordingly, the updated ESC guidelines recommend LAA occlusion in patients with AF who are at a high risk of stroke and who have contraindications to long-term OAC therapy.10
In future studies, it will be important to identify patients who may benefit the most from percutaneous LAA closure as a valid strategy for stroke prevention. It has been reported that LAA closure has a larger ‘net clinical benefit’ when compared with OAC therapy in patients with a high stroke risk—the higher the stroke risk, the larger the ‘net clinical benefit’ of LAA occlusion.26 On the other hand, patients at a high risk for thromboembolism—high platelet count, high CHADS2 score, LV dysfunction or prior stroke—have also been shown to be at a higher risk for LA cavity thrombus27 and device thrombosis.57 Clearly, more studies are warranted to investigate whether some subgroups are more suitable for LAA closure than others.
When starting up an LAA closure programme, it is important to understand that safety is the most ‘vulnerable’ aspect of the procedure—a low complication rate is a ‘must’ in order to establish a successful programme. Moreover, one should realise that imaging is an integral part of planning, performing and follow-up of LAA closure procedures. In addition, the LAA has a highly variable anatomical structure and may offer various procedural challenges.
Finally, we believe that future studies should attempt to (1) further refine the selection of patients most suitable for LAA closure, (2) explore the usefulness of a more tailored postprocedural antithrombotic therapy—adapted to the individual thrombotic and bleeding risk profile, (3) investigate the prevalence and impact of peridevice leaks and device thrombosis in larger cohorts and (4) contribute to further design improvement(s) of LAA closure devices.
Conclusion
Percutaneous LAA occlusion has been shown to be a safe, efficacious and cost-effective strategy67 for stroke prevention in patients with NVAF with increased stroke and bleeding risk. However, since these high-risk patients were typically excluded or under-represented in the available RCTs, additional comparative studies of percutaneous LAA closure versus OAC therapy are needed before this procedure can be recommended for clinical routine.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online supplement
Footnotes
-
Contributors ODB collected and analysed all data and wrote the first draft of the manuscript. OWF contributed essentially to the content of the article. SA, NI, NV, EJ, SP, TDWK, PM and LS revised the manuscript critically and approved the final version for publication. OWF is the guarantor.
-
Funding Grant funding (St Jude Medical) for research.
-
Competing interests None.
-
Provenance and peer review Not commissioned; externally peer reviewed.
-
Data sharing statement No additional data are available.