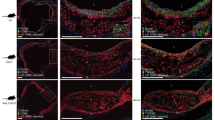
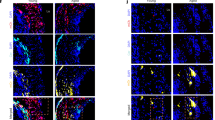
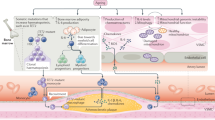
Abstract
During progression of atherosclerosis, myeloid cells destabilize lipid-rich plaques in the arterial wall and cause their rupture, thus triggering myocardial infarction and stroke. Survivors of acute coronary syndromes have a high risk of recurrent events for unknown reasons. Here we show that the systemic response to ischaemic injury aggravates chronic atherosclerosis. After myocardial infarction or stroke, Apoe−/− mice developed larger atherosclerotic lesions with a more advanced morphology. This disease acceleration persisted over many weeks and was associated with markedly increased monocyte recruitment. Seeking the source of surplus monocytes in plaques, we found that myocardial infarction liberated haematopoietic stem and progenitor cells from bone marrow niches via sympathetic nervous system signalling. The progenitors then seeded the spleen, yielding a sustained boost in monocyte production. These observations provide new mechanistic insight into atherogenesis and provide a novel therapeutic opportunity to mitigate disease progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Goldstein, J. A. et al. Multiple complex coronary plaques in patients with acute myocardial infarction. N. Engl. J. Med. 343, 915–922 (2000)
Milonas, C. et al. Effect of angiotensin-converting enzyme inhibition on one-year mortality and frequency of repeat acute myocardial infarction in patients with acute myocardial infarction. Am. J. Cardiol. 105, 1229–1234 (2010)
Libby, P., Ridker, P. M. & Hansson, G. K. Progress and challenges in translating the biology of atherosclerosis. Nature 473, 317–325 (2011)
Weber, C. & Noels, H. Atherosclerosis: current pathogenesis and therapeutic options. Nature Med. 17, 1410–1422 (2011)
Randolph, G. J. The fate of monocytes in atherosclerosis. J. Thromb. Haemost. 7 (suppl. 1). 28–30 (2009)
Charo, I. F. & Ransohoff, R. M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354, 610–621 (2006)
Galkina, E. & Ley, K. Immune and inflammatory mechanisms of atherosclerosis. Annu. Rev. Immunol. 27, 165–197 (2009)
Ernst, E., Hammerschmidt, D. E., Bagge, U., Matrai, A. & Dormandy, J. A. Leukocytes and the risk of ischemic diseases. J. Am. Med. Assoc. 257, 2318–2324 (1987)
Sabatine, M. S. et al. Relationship between baseline white blood cell count and degree of coronary artery disease and mortality in patients with acute coronary syndromes: a TACTICS-TIMI 18 substudy. J. Am. Coll. Cardiol. 40, 1761–1768 (2002)
Nahrendorf, M. et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047 (2007)
Nahrendorf, M., Pittet, M. J. & Swirski, F. K. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 121, 2437–2445 (2010)
Massa, M. et al. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood 105, 199–206 (2005)
Galis, Z. S., Sukhova, G. K., Lark, M. W. & Libby, P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J. Clin. Invest. 94, 2493–2503 (1994)
Libby, P. Inflammation in atherosclerosis. Nature 420, 868–874 (2002)
Chen, J. et al. In vivo imaging of proteolytic activity in atherosclerosis. Circulation 105, 2766–2771 (2002)
Nahrendorf, M. et al. Hybrid in vivo FMT-CT imaging of protease activity in atherosclerosis with customized nanosensors. Arterioscler. Thromb. Vasc. Biol. 29, 1444–1451 (2009)
Tacke, F. et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 117, 185–194 (2007)
Swirski, F. K. et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 117, 195–205 (2007)
Robbins, C. S. et al. Extramedullary hematopoiesis generates Ly-6Chigh monocytes that infiltrate atherosclerotic lesions. Circulation 125, 364–374 (2012)
Leuschner, F. et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J. Exp. Med. 209, 123–137 (2012)
Swirski, F. K. et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 (2009)
Psaltis, P. J. et al. Identification of a monocyte-predisposed hierarchy of hematopoietic progenitor cells in the adventitia of postnatal murine aorta. Circulation 125, 592–603 (2012)
Kondo, M. et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu. Rev. Immunol. 21, 759–806 (2003)
Geissmann, F. et al. Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 (2010)
Zigmond, R. E. & Ben-Ari, Y. Electrical stimulation of preganglionic nerve increases tyrosine hydroxylase activity in sympathetic ganglia. Proc. Natl Acad. Sci. USA 74, 3078–3080 (1977)
Katayama, Y. et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124, 407–421 (2006)
Méndez-Ferrer, S. et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834 (2010)
Méndez-Ferrer, S., Lucas, D., Battista, M. & Frenette, P. S. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452, 442–447 (2008)
Cannon, C. P. et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N. Engl. J. Med. 350, 1495–1504 (2004)
Hoffmann, C., Leitz, M. R., Oberdorf-Maass, S., Lohse, M. J. & Klotz, K. N. Comparative pharmacology of human β-adrenergic receptor subtypes—characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch. Pharmacol. 369, 151–159 (2004)
Kruszewska, B., Felten, S. Y. & Moynihan, J. A. B. Alterations in cytokine and antibody production following chemical sympathectomy in two strains of mice. J. Immunol. 155, 4613–4620 (1995)
Lo Celso, C. et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature 457, 92–96 (2009)
Ding, L., Saunders, T. L., Enikolopov, G. & Morrison, S. J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462 (2012)
Williams, D. A., Rios, M., Stephens, C. & Patel, V. P. Fibronectin and VLA-4 in haematopoietic stem cell–microenvironment interactions. Nature 352, 438–441 (1991)
Lo Celso, C. & Scadden, D. T. The haematopoietic stem cell niche at a glance. J. Cell Sci. 124, 3529–3535 (2011)
Libby, P. & Theroux, P. Pathophysiology of coronary artery disease. Circulation 111, 3481–3488 (2005)
Acknowledgements
We thank the CSB Mouse Imaging Program (J. Truelove, D. Jeon, J. Donahoe, B. Marinelli) and K. Naxerova for helpful discussions. This work was funded by grants from the National Institute of Health R01-HL096576, R01-HL095629 (M.N.); R01-EB006432, T32-CA79443, P50-CA086355 (R.W.). F.L. was funded in part by Deutsche Forschungsgemeinschaft SFB 938/Z2. Fig. 5e was produced using Servier Medical Art (http://www.servier.com).
Author information
Authors and Affiliations
Contributions
P.D. and G.C. performed experiments, collected and analysed the data, and contributed to writing the manuscript, R.G. did surgeries and performed experiments, Y.W., F.Le., R.G., C.S.R., Y.I., B.T., A.L.C., T.H., M.D.M., F.La., M.E., P.W., M.T.W., A.T.C., A.M.v.d.L., H.W.M.N., J.J.P., B.B.R., J.B., J.R.S., H.A.K., C.V., S.A.M., D.A.M. and M.S.S. performed experiments, collected, analysed and discussed data, M.A.M., M.J.P., P.L., C.P.L., F.K.S. and R.W. conceived experiments and discussed strategy and results; M.N. designed and managed the study and wrote the manuscript, which was edited and approved by all co-authors.
Corresponding authors
Ethics declarations
Competing interests
M.S.S., D.A.M. and S.A.M. received grant support from AstraZeneca and GSK. The remaining authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-29, Supplementary Table 1, Supplementary Methods and Supplementary References. (PDF 5464 kb)
Rights and permissions
About this article
Cite this article
Dutta, P., Courties, G., Wei, Y. et al. Myocardial infarction accelerates atherosclerosis. Nature 487, 325–329 (2012). https://doi.org/10.1038/nature11260
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11260
This article is cited by
-
Role of CD4+ T-cells for regulating splenic myelopoiesis and monocyte differentiation after experimental myocardial infarction
Basic Research in Cardiology (2024)
-
Axin2 depletion in macrophages alleviated senescence and increased immune response after myocardial infarction
Inflammation Research (2024)
-
Reduced hematopoietic-inflammatory response and worse outcomes in patients with recurrent myocardial infarction in comparison with primary myocardial infarction
EJNMMI Research (2023)
-
Establishment and validation of a prediction nomogram for heart failure risk in patients with acute myocardial infarction during hospitalization
BMC Cardiovascular Disorders (2023)
-
Triage body temperature and its influence on patients with acute myocardial infarction
BMC Cardiovascular Disorders (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.