Article Text
Abstract
Background Dual antiplatelet therapy is the standard of care after coronary stent placement but increases the bleeding risk. The effects of proton pump inhibitors (PPIs) on clopidogrel metabolism have been described, but the clinical significance is not yet definitive. We aimed to do an updated meta-analysis comparing outcomes in patients receiving clopidogrel with and without PPIs.
Methods We systematically searched PubMed, Scopus and the Cochrane Central Register of Controlled Trials for randomised controlled trials (RCTs) and controlled observational studies in patients taking clopidogrel stratified by concomitant PPI use. Heterogeneity was examined with the Cochran Q test and I2 statistics; p values inferior to 0.10 and I2 >25% were considered significant for heterogeneity.
Results We included 39 studies with a total of 214 851 patients, of whom 73 731 (34.3%) received the combination of clopidogrel and a PPI. In pooled analysis, all-cause mortality, myocardial infarction, stent thrombosis and cerebrovascular accidents were more common in patients receiving both drugs. However, among 23 552 patients from eight RCTs and propensity-matched studies, there were no significant differences in mortality or ischaemic events between groups. The use of PPIs in patients taking clopidogrel was associated with a significant reduction in the risk of gastrointestinal bleeding.
Conclusions The results of our meta-analysis suggest that PPIs are a marker of increased cardiovascular risk in patients taking clopidogrel, rather than a direct cause of worse outcomes. The pharmacodynamic interaction between PPIs and clopidogrel most likely has no clinical significance. Furthermore, PPIs have the potential to decrease gastrointestinal bleeding in clopidogrel users.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Key messages
What is already known about this subject?
Proton pump inhibitors have the potential to decrease the risk of upper gastrointestinal haemorrhage among patients taking antiplatelet therapy. However, pharmacokinetic data and observational studies have suggested a potential interaction between clopidogrel and proton pump inhibitors, which could have a significant effect in clinical events.
What does this study add?
We studied potential factors associated with the interaction between clopidogrel and proton pump inhibitors, such as stent placement, presentation as an acute coronary syndrome, use of dual antiplatelet therapy, and stratification by different proton pump inhibitors. Importantly, in a subanalysis of studies with randomised or propensity score matched data, no significant difference was observed in adverse outcomes between patients who received a proton pump inhibitor and those who did not. The reduction in gastrointestinal bleeding among patients taking a proton pump inhibitor was consistent throughout the different subgroups.
How might this impact on clinical practice?
The results of our study suggest that the previously reported interaction between clopidogrel and proton pump inhibitors may be dependent on selection bias and different patient baseline characteristics, as a clinically significant effect was not observed in a randomised/propensity score matched population. On the basis of these findings, physicians may consider proton pump inhibitors for patients receiving clopidogrel, as there is a benefit in terms of reduced gastrointestinal bleeding.
Introduction
Dual antiplatelet therapy (DAPT) with aspirin and clopidogrel is recommended following acute coronary syndromes (ACS) and percutaneous coronary interventions (PCI), as it has been shown to decrease the risk of adverse cardiovascular (CV) events.1–5 PPIs significantly decrease the risk of upper gastrointestinal (GI) haemorrhage in patients receiving antiplatelet therapy.6–8
Clopidogrel activation is dependent on the hepatic cytochrome P450, which can be competitively inhibited by PPIs.9–12 The potential interaction between clopidogrel and PPIs has been extensively demonstrated in pharmacokinetic platelet aggregation studies.13–16 These findings led to label warnings from the Food and Drug Administration regarding the concomitant use of clopidogrel with omeprazole or esomeprazole.17 Furthermore, these concerns have resulted in more restricted guideline indications for PPIs in patients taking antiplatelet therapy.18
Nevertheless, the majority of data on the clinical significance of the PPI-clopidogrel interaction derive from observational studies and the results have been conflicting.19–23 Two randomised controlled trials (RCTs) have failed to show an increased incidence of ischaemic CV outcomes in patients on concomitant use of clopidogrel and a PPI.7 ,24 Multiple meta-analyses have been performed, but the most recent one included data only until June 2012.25–29 A substantial number of studies have been published since then, including over 50 000 patients.30–36 We aimed to perform an updated meta-analysis comparing the incidence of adverse CV and GI events in patients receiving clopidogrel with and without PPIs. Furthermore, we sought to identify possible factors in the clopidogrel-PPI interaction, such as ACS, DAPT and specific PPIs.
Material and methods
Eligibility criteria and data extraction
We restricted our analysis to studies that met all the following inclusion criteria: (1) RCTs, case–control or cohort (retrospective or prospective) studies; (2) patients on clopidogrel stratified into two groups: concomitant PPI-clopidogrel use versus clopidogrel use alone; (3) available data on any of the outcomes of interest in a direct comparison between PPI and non-PPI users; and (4) at least 6 months of follow-up. Exclusion criteria were non-controlled studies (absence of comparison group on clopidogrel without concomitant PPI use), ongoing studies and duplicate reports. In studies with outcomes reported in person-years rather than in absolute values, we attempted contact with the authors to obtain patient-level data.
Each of the four authors (RNC, DCG, FYBM, GEH) independently extracted data following the defined search criteria and quality assessment. Disagreements between these four authors were resolved by consensus. In addition to outcomes of interest, the authors also extracted further information for subgroup analyses, including population characteristics, specific PPI used, concomitant use of aspirin and study design.
Search strategy
We systematically searched PubMed, Scopus and the Cochrane Central Register of Controlled Trials for RCTs and controlled observational studies in patients taking clopidogrel stratified by concomitant PPI use. The search was conducted without date restrictions in February 2014 for studies published in English only. The following medical subject heading terms were included: (clopidogrel OR Plavix) AND (PPI OR proton pump inhibitor OR omeprazole OR esomeprazole OR rabeprazole OR pantoprazole OR lansoprazole OR ilaprazole OR dexlansoprazole). In addition to searching databases, investigators also reviewed abstracts from the main cardiology and GI conferences from 2010 to 2014. Reference lists of all included studies, meta-analysis and reviews were manually searched. There was no patient population size restriction for the search.
End points and subgroup analyses
Outcomes of interest included all-cause mortality, CV mortality, myocardial infarction (MI), ACS, stent thrombosis, revascularisation, cerebrovascular accidents (CVA) and GI bleeding. Given the large number of studies and availability of individual outcomes, combined end points were not used. For the outcome of stent thrombosis, thought to be the most prone to variability in definitions, a subanalysis was performed including only definite cases according to Academic Research Consortium criteria.37 Owing to an anticipated variability in the definitions of GI bleeding, we restricted our analysis to gastric or duodenal bleeding confirmed by endoscopy.
In the search for potential factors associated with a clopidogrel-PPI interaction, prespecified subgroup analyses were performed. These included (1) concomitant treatment with aspirin (DAPT); (2) patients with PCI; (3) patients with ACS; and (4) stratification by risk of clopidogrel interaction according to degree of CYP450 2C19 inhibition. The high-risk PPI group included omeprazole, esomeprazole and lansoprazole, which are considered the most prone to CYP450 2C19 inhibition.11 ,16 ,17 ,20 Pantoprazole and rabeprazole were analysed separately in the low-risk PPI group.16 ,20 ,27 ,38 Finally, a subanalysis was also performed that was restricted to RCTs and propensity score matched (PSM) studies to evaluate for the possibility of selection bias in observational studies.
Quality assessment
The quality of case–control and cohort studies was evaluated by the Newcastle-Ottawa Scale (NOS).39 This tool for quality assessment of non-randomised studies attributes none to nine stars according to the methodological quality of three parameters: selection of participants; comparability of groups; and assessment of either exposure in case–controls or outcomes in cohort studies. Previous meta-analyses have considered studies with 6 or more stars as high quality.40 ,41 Post hoc analyses of RCTs were assessed as cohort studies by the NOS, given that the exposure of interest was not a randomised factor. With the exception of the two conference abstracts, all case–control and cohort studies included received a score of 7 or higher on the Newcastle-Ottawa scale and therefore were considered studies of high methodological quality. Quality assessment of RCTs was performed with the Jadad score, which evaluates randomisation, blinding and follow-up.42 Publication bias was evaluated by using funnel-plot graphs and checking for symmetrical distribution of trials with similar weights.43
Statistical analysis
Meta-analysis was performed according to recommendations of the Cochrane Collaboration and in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement.44 Pooled treatment effects were estimated using OR with 95% CIs for binary end points. We used the random-effects DerSimonian and Laird model because of the anticipated wide variability between studies, particularly among observational data. Nevertheless, results were confirmed with the Mantel-Haenszel fixed-effect model to avoid small studies being overly weighted. Heterogeneity was examined with the Cochran Q test and I2 statistics; p values inferior to 0.10 and I2 >25% were considered significant for heterogeneity.45 For statistical analysis, we used Review Manager 5.1 (Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
Results
Study selection and characteristics
As illustrated in figure 1, overall 2125 studies were identified. After removal of duplicate reports, animal studies and non-relevant studies by title or abstract review, 93 articles remained. These were fully reviewed for satisfaction of inclusion criteria. The main reasons for withdrawal were absence of control group, outcomes of interest not reported or a short follow-up interval.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram of study selection.
Thirty-seven manuscripts met all criteria and were included. An additional two studies were included from a review of conference abstracts. A total of 39 studies and 214 851 patients were included, of whom 73 731 (34.3%) received the combination of clopidogrel and a PPI. The vast majority were observational studies and only three RCTs were identified. Study characteristics are presented in table 1. Baseline characteristics in individual studies were most commonly not comparable between groups; therefore, a subanalysis of RCTs/propensity score matched (PSM) populations was carried out to evaluate the impact of selection bias in study results.
Characteristics of studies included in meta-analysis
Pooled analysis of all studies
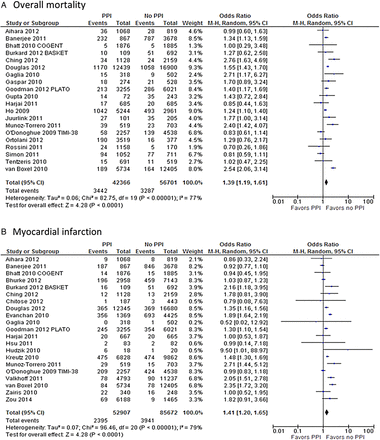
All-cause mortality (OR 1.39; 95% CI 1.19 to 1.61; p<0.001) and MI (OR 1.41; 95% CI 1.20 to 1.65; p<0.001) were significantly increased in the group of patients receiving PPIs, as illustrated in figure 2. Stent thrombosis (OR 1.30; 95% CI 1.05 to 1.63; p=0.02), definite stent thrombosis (OR 1.65; 95% CI 1.10 to 2.48; p=0.02; figure 3A), ACS (OR 1.92; 95% CI 1.23 to 3.0; p=0.004; figure 3B) and CVA (OR 1.66; 95% CI 1.40 to 1.97; p<0.001; figure 3C) were also more common in patients receiving both drugs. There was also a strong trend towards increased revascularisation (OR 1.26; 95% CI 1.0 to 1.59; p=0.05; figure 3D) in the PPI-clopidogrel group. Conversely, the concomitant use of PPIs had a protective effect over the risk of GI bleeding (OR 0.40; 95% CI 0.22 to 0.74; p=0.003; figure 4). A separate analysis restricted to cohort studies revealed similar results to the overall analysis.
Forest plot of studies examining outcomes between patients taking proton pump inhibitor ( PPIs) with clopidogrel and those taking only clopidogrel: (A) overall mortality; (B) myocardial infarction.
Forest plot of studies examining outcomes between patients taking proton pump inhibitor (PPIs) with clopidogrel and those taking only clopidogrel: (A) definite stent thrombosis; (B) need for revascularisation; (C) cerebrovascular accidents; (D) acute coronary syndromes.
Forest plot of studies examining gastrointestinal bleeding between patients taking proton pump inhibitor (PPI) with clopidogrel and those taking only clopidogrel.
RCTs and propensity score matched studies
Given the overwhelming majority of non-randomised observational studies in our meta-analysis and the subsequent risk of baseline heterogeneity between groups, a subanalysis was performed including only RCTs and PSM populations. A total of 23 552 patients were entered in the analysis, of whom 11 770 (49.9%) received the combination of clopidogrel and a PPI. Results are illustrated in figure 5 and show that all-cause mortality (OR 0.91; 95% CI 0.58 to 1.40; p=0.66), ACS (OR 0.96; 95% CI 0.88 to 1.05; p=0.35), MI (OR 1.05; 95% CI 0.86 to 1.28; p=0.65) and CVA (OR 1.47; 95% CI 0.66 to 3.25; p=0.34) were not significantly different between treatment groups. Revascularisation (OR 0.88; 95% CI 0.80 to 0.97; p=0.01) was also not increased in patients who received concomitant PPI with clopidogrel. Furthermore, occurrence of GI bleed was significantly decreased in the group of patients who received a PPI (OR 0.24; 95% CI 0.09 to 0.62; p=0.003).
Forest plots of randomised controlled trials and propensity score matched studies examining outcomes between patients taking proton pump inhibitor (PPIs) with clopidogrel and those taking only clopidogrel: (A) overall mortality; (B) acute coronary syndromes; (C) myocardial infarction; (D) need for revascularisation; (E) cerebrovascular accidents; (F) gastrointestinal bleeding.
Subgroup analyses
Table 2 illustrates results of comparisons in studies with restricted populations. In studies limited to patients with ACS, only MI (OR 1.41; p=0.01) was significantly increased in patients taking clopidogrel with a concomitant PPI. In patients receiving DAPT, adding a PPI decreased the risk of an upper GI bleed (OR 0.31; p=0.002), but was associated with increased risk of all-cause mortality (OR 1.32; p=0.003), ACS (OR 2.37; p=0.002), MI (OR 1.25; p=0.005), stent thrombosis (OR 1.36; p=0.005) and revascularisation (OR 1.30; p=0.006). Stratification by degree of CYP450 2C19 inhibition revealed that both high-risk (omeprazole, esomeprazole and lansoprazole) and low-risk PPIs (pantoprazole and rabeprazole) were associated with an increased risk of MI and mortality. In patients receiving high-risk PPIs, GI bleed was also decreased by concomitant PPI use (OR 0.17; p<0.001).
Subgroup analyses
Quality assessment
One of the RCTs included was stopped prematurely due to a loss of funding.7 Nevertheless, it was considered a high quality study according to the Jadad criteria. The other two RCTs were considered to be of moderate quality because blinding was not described.24 On funnel plot analysis, studies occupied a symmetrical distribution according to weight and converged towards the pooled effect as the weight increased (see online supplementary figure S1). Egger's regression test (see online supplementary figure S2) was also performed and showed no evidence of significant publication bias (p=0.48 and 0.76 for overall mortality and MI, respectively).
Discussion
The pooled analysis of all included studies included 214 851 patients and found that patients who took a PPI in addition to clopidogrel had the worst outcomes, including higher overall mortality, MI, ACS, CVA, stent thrombosis and the need for revascularisation procedures. These results are consistent with previous studies and meta-analyses.26 ,48 ,51 ,67 However, these data emerge mostly from nonrandomised observational studies, which are prone to selection bias and non-comparability between groups at baseline. Therefore, we conducted a separate analysis including data only from RCTs and PSM patients. In a population of 23 552 patients from eight studies, we found that all ischaemic end points evaluated were not increased in the clopidogrel-PPI group (figure 5). This analysis of RCTs and PSM patients highly suggests that PPIs are a marker of increased risk, rather than a direct cause of worse outcomes.
The contrast in outcomes between unadjusted and adjusted/randomised studies is supported by findings of increased CV risk among patients taking PPI regardless of simultaneous clopidogrel use. In a population of 31 704 patients who were not receiving clopidogrel, Charlot et al73 found that, compared with non-PPI users, patients on PPI had an increased risk of all-cause mortality (HR 1.58; p<0.01), CV mortality (HR 1.49; p<0.01), MI (HR 1.13; p=0.02) and CVA (HR 1.32; p<0.01). Furthermore, the magnitude of increased CV risk in the PPI group was similar between clopidogrel users and patients not receiving clopidogrel. An increased risk of ischaemic outcomes among patients taking a PPI has also been reported in concomitant use of placebo and ticagrelor.68 ,74
The mechanism of increased CV risk in patients receiving a PPI is most likely related to the difference in baseline characteristics between users and non-users of clopidogrel. In the study by Charlot et al,73 patients who received a PPI were on average 3 years older than the comparison group and also had a higher prevalence of diabetes with complications, chronic kidney injury and cerebrovascular disease at baseline. In Bhurke et al,30 patients taking clopidogrel had a higher Charlson comorbidity index at baseline, as well as a higher prevalence of heart failure. Similarly, the majority of unadjusted studies that reported an increased risk of CV events in PPI users had an unbalanced distribution of baseline characteristics, with sicker patients in the PPI group.31 ,52 ,63 ,67
Our study found a decreased incidence of GI bleeding among patients taking PPIs, a result that was confirmed in patients with similar baseline characteristics (RCT/PSM populations; figure 5F). Two different mechanisms may contribute as follows to the decreased incidence of GI bleeding with PPI use. The first is by direct inhibition of proton pumps with subsequent suppression of acid production, which has been shown to (1) prevent stress-ulcer related bleeding in critically ill patients;75 (2) decrease rebleeding in patients with a history of ulcer-related bleeding;6 and (3) decrease GI bleeding among patients on anticoagulants and dual anti-platelet therapy.76 Alternatively, the benefit in GI bleeding may be related to a PPI-mediated reduction in the antiplatelet effect of clopidogrel. Several pharmacokinetic studies have demonstrated a lower inhibition of platelet aggregation among patients taking a PPI in addition to clopidogrel, as compared to non-PPI clopidogrel users.14 ,15 ,66 Although our findings suggest that this mechanism is not clinically relevant in terms of adverse CV outcomes, platelet aggregation plays an important role in angiogenesis and the healing of peptic ulcers;77 therefore, a lesser degree of platelet inhibition certainly has the potential to decrease GI bleeding.
As illustrated in table 2, among patient with ACS, there was no increased risk of ischaemic CV end points with PPI use. Patients with ACS most likely have more comorbidities and a worse prognosis at baseline compared with elective patients, which can mitigate the differences in outcomes between PPI and non-PPI users. Inhibition of the CYP450 2C19 enzyme is heterogeneous within the class of PPIs. Omeprazole, esomeprazole and lansoprazole have been shown to be the strongest inhibitors,11 ,16 ,17 ,20 whereas some studies have suggested that pantoprazole and rabeprazole have no effect on the CYP450 2C19 enzyme.16 ,20 ,38 Our meta-analysis has demonstrated that the association between adverse outcomes and concomitant PPI-clopidogrel use persists in patients taking the low-risk PPIs rabeprazole or pantoprazole. Given that these medications are not expected to have a significant interaction with clopidogrel, this finding further supports the hypothesis that use of a PPI is not the cause of increased adverse outcomes, but rather a marker of increased baseline risk.
This study has limitations. Definitions of outcomes were not reported in a substantial part of the studies, which raises the concern for reporting bias. In addition, 36 of the 39 included studies were non-randomised and are inherently more susceptible to bias. The correction of possible baseline differences between groups led to a subanalysis of randomised and PSM studies; however, this analysis included only eight studies, which did not report on all the studied outcomes. Moreover, the absence of patient-level data, common in meta-analysis designs, prevented more detailed subgroup analyses, such as interaction between different generations of drug-eluting stents and the exact role of baseline characteristics on the clopidogrel-PPI interaction. Also, this systematic review was not registered prospectively, which would have allowed feedback about the protocol, further limiting the possibility of bias. Nevertheless, we believe we have conducted a transparent and reproducible protocol. Finally, given the high number of studies included and the differences in methods and outcome definitions among them, a substantial amount of heterogeneity was encountered. This has already been observed in previous meta-analyses, and therefore only a random-effects model was used. A prespecified definition of GI bleeding and stent thrombosis was also applied to minimise bias resulting from different outcome definitions.
Conclusion
In summary, the results of our meta-analyses suggest that the highly controversial interaction between PPIs and clopidogrel observed in platelet aggregation studies has no clinical significance. Rather, patients who are prescribed PPIs have a higher burden of comorbidities and thus most likely have an increased risk for adverse CV events. Importantly, PPIs have the potential to significantly reduce GI bleeding among patients taking clopidogrel.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1 - Online figures
Footnotes
RNC and AMB contributed equally and share first authorship.
Contributors AMB, JPR were involved in the conception and design of the study; analysis and interpretation of data and drafting of the manuscript; final approval of the manuscript submitted. RNC was involved in the conception and design of the study; data collection and drafting of the manuscript; final approval of the manuscript submitted. JJD, GNN were involved in the analysis and interpretation of data; critical revision of the manuscript for important intellectual content; final approval of the manuscript submitted. DCG, FYBM, GE-H were involved in the data collection; final approval of the manuscript submitted. IK was involved in the analysis and interpretation of data; final approval of the manuscript submitted. SG, MI were involved in the statistical analysis; final approval of the manuscript submitted.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement No additional data are available.