Article Text
Abstract
Background Cilostazol overcomes high on-treatment platelet reactivity (HTPR) and reduces adverse cardiovascular (CV) outcomes after percutaneous coronary intervention (PCI). However, the role for triple antiplatelet therapy (TAPT) with cilostazol in addition to aspirin and clopidogrel after PCI is not well defined.
Methods We conducted a MEDLINE/EMBASE/CENTRAL search for randomised trials, until May 2014, evaluating TAPT compared with dual antiplatelet therapy (DAPT) of aspirin and clopidogrel alone in patients undergoing PCI and reporting platelet reactivity and/or CV outcomes. The primary platelet reactivity outcome was differences in platelet reactivity unit (PRU) with secondary outcomes of %platelet inhibition and rate of HTPR. The primary CV outcome was major adverse cardiovascular events (MACE), with secondary outcomes of death, cardiovascular death, myocardial infarction, stent thrombosis (ST), target lesion revascularisation (TLR) and target vessel revascularisation (TVR) as well as safety outcomes of bleeding and drug discontinuations.
Results In 17 trials that evaluated platelet reactivity outcomes, the mean PRU value was 47.73 units lower with TAPT versus DAPT (95% CI −61.41 to −34.04, p<0.0001; mean PRU 182.90 vs 232.65). TAPT also increased platelet inhibition by 12.71% (95% CI 10.76 to 14.67, p<0.0001), and led to a 60% reduction in the risk of HTPR (relative risk=0.40; 95% CI 0.30 to 0.53) compared with DAPT. Moreover, among the 34 trials that evaluated CV outcomes, TAPT reduced the risk of MACE (incident rate ratio (IRR)=0.68; 95% CI 0.60 to 0.78), TLR (IRR=0.57; 95% CI 0.44 to 0.73), TVR (IRR=0.69; 95% CI 0.59 to 0.81) and ST (IRR=0.63; 95% CI 0.40 to 0.98) with no difference for other outcomes including bleeding, even in trials using drug-eluting stents. Drug discontinuation due to adverse effects was, however, higher with TAPT vs DAPT (IRR=1.59; 95% CI 1.32 to 1.91).
Conclusions In patients undergoing PCI, addition of cilostazol to DAPT results in decreased platelet reactivity and a significant reduction in CV outcomes including ST, even in the drug-eluting stent era.
- CORONARY ARTERY DISEASE
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/
Statistics from Altmetric.com
Key messages
-
What is already known about this subject?
-
Cilostazol, a phosphodiesterase III inhibitor, exhibits antiplatelet effect and inhibits neointimal hyperplasia and smooth muscle proliferation. However, its role in addition to dual antiplatelet therapy (DAPT) of aspirin and clopidogrel in patients undergoing percutaneous coronary intervention (PCI) is not well defined.
-
What does this study add?
-
In patients undergoing PCI, addition of cilostazol to DAPT results in decreased platelet reactivity and a significant reduction in cardiovascular outcomes including stent thrombosis, even in the drug-eluting stent era.
-
How might this impact on clinical practice?
-
The current study provides evidence to support use of cilostazol as an attractive and strong competitor for newer antiplatelet regimens and should be evaluated in future trials in patients undergoing PCI.
Introduction
Dual antiplatelet therapy (DAPT) with aspirin and an ADP receptor inhibitor is the standard of care for patients undergoing percutaneous coronary intervention (PCI). However, there is significant interindividual variability in the extent of platelet inhibition achieved with clopidogrel.1–3 Several studies have shown a correlation between high levels of on-treatment platelet reactivity (HTPR) and adverse cardiovascular outcomes, such that patients with HTPR (also called clopidogrel resistance) have a threefold to fivefold increased risk for recurrent ischaemic events.4 ,5 Cilostazol, a phosphodiesterase III inhibitor, exhibits its antiplatelet effects via inhibition of the conversion of cyclic AMP (cAMP) to 5'-AMP causing a subsequent increase in cAMP within platelets, and has been shown to augment platelet inhibition when it is added to aspirin and clopidogrel as part of a triple therapy regimen.6 ,7 In addition, cilostazol inhibits neointimal hyperplasia and smooth muscle proliferation, and has the potential to reduce the risk of restenosis after coronary stent implantation.8–11 Despite these pharmacologic effects, clinical results from observational and small randomised trials have not shown a consistent clinical benefit.
Our objective was to evaluate whether triple antiplatelet therapy (TAPT) with cilostazol (in addition to aspirin and clopidogrel) decreases platelet reactivity and reduces adverse cardiovascular (CV) outcomes when compared with a dual antiplatelet (DAPT) regimen of aspirin and clopidogrel alone.
Methods
Eligibility criteria
We conducted a MEDLINE, EMBASE and CENTRAL search using the MeSH terms ‘cilostazol’ and ‘randomised clinical trial’. We limited our search to trials involving human subjects through May 2014. The search terms were broad with no language restrictions imposed. We checked the reference lists of review articles and prior meta-analyses to assess for additional eligible studies. Corresponding authors of studies were contacted for further information if relevant data were not reported. Trials in abstract format without a manuscript published were also included in the analysis.
To be included for analysis, eligible trials had to fulfil the following criteria: (1) randomised clinical trials of TAPT (aspirin, clopidogrel and cilostazol) in comparison to DAPT (aspirin and clopidogrel); (2) enrolment of patients undergoing PCI with drug-eluting or bare metal stents and (3) follow-up of at least 2 weeks for trials reporting platelet reactivity outcomes and at least 1 month for trials reporting cardiovascular outcomes.
Selection and quality assessment
Three authors (AS, BT and SB) independently reviewed trial eligibility and quality. Disagreements were resolved by consensus. Risk of bias was assessed using criteria recommended by the Cochrane Collaboration, specifically evaluating sequence generation of allocation; allocation concealment; blinding of participants, staff and outcome assessors; incomplete outcome data; selective outcome reporting; and other sources of bias.12 Trials with high or unclear risk of bias for the first three criteria were considered as high bias risk trials and the rest as low bias risk trials.
Data extraction and synthesis
The primary platelet reactivity outcome was differences in platelet reactivity unit (PRU) after treatment in TAPT versus DAPT groups. Secondary outcomes were percent platelet inhibition and rate of HTPR. We used a cut-off of PRU >235 as the threshold for identifying patients with HTPR who may be at high risk for ischaemic or thrombotic events following PCI, as has been recommended by a recent consensus document.13 Of note, definition of HTPR differed by study.
Our primary CV outcome was major adverse cardiovascular events (MACE), defined as death, myocardial infarction (MI) or target lesion revascularisation (TLR). We evaluated secondary CV outcomes of death, cardiovascular death, MI, stent thrombosis, TLR and target vessel revascularisation (TVR). Safety outcomes of major bleeding, minor bleeding, any (major or minor) bleeding and drug discontinuation due to adverse effects were also evaluated. The definitions of bleeding varied between the trials. Given the lack of consistent reporting of the Academic Research Consortium definitions of stent thrombosis from the studies, we used the individual trial protocol definitions of stent thrombosis.
Statistical analysis
We performed an intention to treat meta-analysis in line with recommendations from the Cochrane Collaboration and the PRISMA Statement14 ,15 and used standard software for statistical analysis (STATA V.9.0, STATA Corp, Texas, USA). Heterogeneity was assessed using the I2 statistic, defined as the proportion of total variation observed between the trials attributable to differences between trials rather than sampling error (chance), with values <25% considered as low and >75% as high.16 The pooled effect for each grouping of trials was derived from the point estimate for each separate trial weighted by the inverse of the variance (1/SE2). Continuous variable outcomes (PRU, per cent platelet inhibition) between the groups were compared with both a fixed effect model using the inverse variance method and a random effects model using the DerSimonian and Laird method. For cardiovascular outcomes, rates were expressed per patient-years to adjust for the varying duration of follow-up. Results were therefore reported as incident rate ratios (IRR) and 95% CIs with the use of both a fixed effect model using the method of Mantel and Haenszel and a random effects model using the method of DerSimonian and Laird, with the estimate of heterogeneity being taken from the Mantel-Haenszel model. Publication bias was estimated using the weighted regression tests of Begg and Egger.12
For platelet reactivity indices, analyses were stratified based on whether standard-dose (75 mg) or high-dose (150 mg) clopidogrel was used in the DAPT arm. In addition, further sensitivity analyses were performed based on the cohort enrolled: (1) acute coronary syndrome (ACS) versus not; and (2) enrolment of patients with HTPR at baseline versus not. For cardiovascular outcomes, analyses were stratified based on stent type—drug eluting stent (DES) versus Bare metal stent (BMS). A p value of <0.05 was considered significant.
Results
Study selection
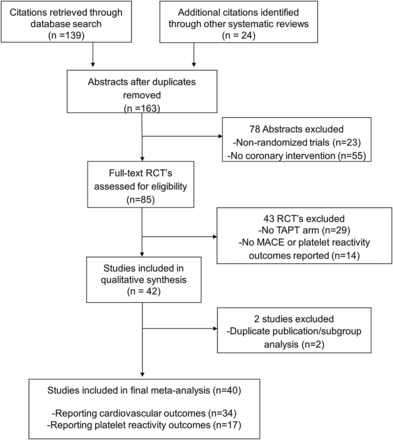
We identified 41 trials that satisfied the inclusion criteria (figure 1). Seventeen trials reported platelet reactivity outcomes of which 10 comparator arms used high dose (150 mg) of clopidogrel. A total of 34 trials reported CV outcomes, the majority (25 trials) of which used DES.
Study selection.
Baseline characteristics
The baseline characteristics, inclusion criteria and quality assessment are summarised in tables 1⇓⇓–4. In order to quantify platelet reactivity outcomes, we evaluated 17 trials with 20 comparator arms and 5056 patients. The median follow-up was 30 days and although the definition of HTPR was heterogeneous, all trials used the VerifyNow P2Y12 assay to measure platelet reactivity. The analysis of cardiovascular outcomes included 34 trials with 14 119 patients. The mean age of study participants was between 56.3 and 67.5 years, 37.9% of the patients had diabetes and the majority (77.6%) underwent PCI with DES.
Baseline characteristics of included trials for platelet reactivity outcomes
Inclusion criteria and study quality for platelet reactivity outcomes trials
Baseline characteristics of included trials for cardiovascular outcomes
Inclusion criteria and study quality of included cardiovascular outcomes trials
Primary platelet reactivity outcomes
Primary outcome: differences in PRU
TAPT resulted in a mean PRU reduction of 47.73 (95% CI −61.41 to −34.04, p<0.0001; mean PRU 182.90 vs 232.65) compared with DAPT (figure 2A). There was a larger mean difference between the TAPT and DAPT groups when the analysis was restricted to a DAPT group using standard-dose clopidogrel (mean PRU 189.54 vs 255.83) where the PRU value was lower by a mean of 64.10 (95% CI −84.35 to −43.85). Moreover, TAPT was associated with a lower PRU value even when compared with DAPT using high-dose clopidogrel (mean difference of 27.17) (mean PRU 176.27 vs 209.48) (figure 2A). The results were similar when stratified by ACS status (see web appendix figure A1) or by baseline clopidogrel resistance status (see web appendix figure A2). There was moderate-to-high heterogeneity for the above analysis. However, the heterogeneity was reduced in subgroup analysis restricted to comparison with high-dose clopidogrel (figure 2A), in trials enrolling patients with baseline clopidogrel resistance (see web appendix figure A2 and in trials enrolling patients without ACS (see web appendix figure A1).
(A) Primary platelet reactivity outcome: difference in platelet reactivity units (PRU) after treatment between triple antiplatelet therapy (TAPT) versus dual antiplatelet therapy (DAPT). (B) Secondary platelet reactivity outcome: difference in percent platelet inhibition after treatment between TAPT versus DAPT. (C) Secondary platelet reactivity outcome: risk of high on-treatment platelet reactivity (HTPR) after treatment between TAPT versus DAPT.
Continued
In addition, the mean PRU values on treatment in the TAPT group in each of the trials were below a PRU of 235, which has been cited in the literature as the suggested threshold for defining HTPR.13
Secondary outcomes: percent platelet inhibition and high on-treatment platelet reactivity
TAPT was associated with a 12.71% greater platelet inhibition compared to DAPT for the overall cohort (95% CI 10.76 to 14.67, p<0.0001) (figure 2B). TAPT was also associated with a greater platelet inhibition in comparison with DAPT using standard-dose clopidogrel (14.37% mean greater platelet inhibition) and remained significant even when compared with DAPT using high-dose clopidogrel (9.07% mean greater platelet inhibition) (figure 2B). There was moderate heterogeneity for the above analysis. The results were similar when stratified by ACS status (see web appendix figure A3) or by baseline clopidogrel resistance status (see web appendix figure A4).
In addition, TAPT was associated with a 60% reduction in the risk of HTPR when compared with DAPT (figure 2C) (relative risk=0.40; 95% CI 0.30 to 0.53, p<0.0001). When stratified by clopidogrel dose, TAPT was associated with a 50% reduction in risk of HTPR compared to standard-dose DAPT and a 72% reduction compared to high-dose DAPT (figure 2C). Heterogeneity was moderate with no evidence for significant publication bias. The results were similar when stratified by ACS status (see web appendix figure A5) or by baseline clopidogrel resistance status (see web appendix figure A6).
Cardiovascular outcomes
Primary outcome
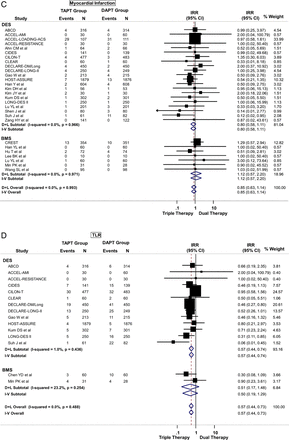
TAPT was associated with a 32% reduction in the risk of MACE (IRR=0.68; 95% CI 0.60 to 0.78) when compared with DAPT for the overall cohort (figure 3A). This effect was observed regardless of stent type (Pinteraction >0.05) such that even in patients undergoing PCI with DES, TAPT resulted in a 36% reduction in MACE (IRR=0.64; 95% CI 0.55 to 0.75) when compared with DAPT alone (figure 3A). There was low heterogeneity in the analysis and no evidence for significant publication bias.
(A) Primary cardiovascular outcome: risk of major adverse cardiovascular effects (MACE) between triple antiplatelet therapy (TAPT) versus dual antiplatelet therapy (DAPT). (B) Secondary cardiovascular outcome: risk of all-cause mortality between TAPT versus DAPT. (C) Secondary cardiovascular outcome: risk of myocardial infarction between TAPT versus DAPT. (D) Secondary cardiovascular outcome: risk of target lesion revascularisation (TLR) between TAPT versus DAPT. (E) Secondary cardiovascular outcome: risk of target vessel revascularisation (TVR) between TAPT versus DAPT. (F) Secondary cardiovascular outcome: risk of stent thrombosis between TAPT versus DAPT.
Continued
Continued
Secondary outcomes
TAPT was associated with similar IRR for death (IRR=0.79; 95% CI 0.58 to 1.09) (figure 3B), cardiovascular death (IRR=0.74; 95% CI 0.42 to 1.30) and MI (IRR=0.85; 95% CI 0.63 to 1.14) (figure 3C) for the overall cohort. The IRR was independent of stent type as TAPT showed benefit regardless whether BMS and DES was used (stent type, Pinteraction >0.05). In the overall cohort, TAPT was associated with a 43% reduction in the risk of TLR (IRR=0.57; 95% CI 0.44 to 0.73) (figure 3D) and a 31% reduction in the risk of TVR (IRR=0.69; 95% CI 0.59 to 0.81) (figure 3E) compared with DAPT. TAPT efficacy for reducing TLR and TVR was present even when the analyses were restricted to studies using DES. In DES-treated patients, TAPT resulted in a 43% reduction in TLR (IRR=0.57; 95% CI 0.44 to 0.74) and a 35% reduction in TVR (IRR=0.65; 95% CI 0.54 to 0.79) with TAPT compared with DAPT.
TAPT was associated with significantly lower stent thrombosis rate when compared with DAPT (IRR=0.63; 95% CI 0.40 to 0.98) (figure 3F). There was no heterogeneity (0%) in all of the above analyses and no evidence for significant publication bias.
Safety outcomes
TAPT was associated with a numerically increased risk of major (IRR=1.24; 95% CI 0.79 to 1.92) (figure 4A), minor (IRR=1.37; 95% CI 0.88 to 2.14) (figure 4B), or any bleeding (IRR=1.26; 95% CI 0.99 to 1.61) (figure 4C) compared with DAPT, although these were not statistically significant. TAPT was also associated with a 59% increase in drug discontinuation due to adverse events (IRR=1.59; 95% CI 1.32 to 1.91) (figure 4D) when compared with DAPT. The most commonly listed causes for drug discontinuation were headache, skin rash and palpitations/tachycardia. There was no-to-modest (for drug discontinuation outcomes) heterogeneity in all of the above analyses and no evidence for significant publication bias.
(A) Safety outcome: risk of major bleeding between triple antiplatelet therapy (TAPT) versus dual antiplatelet therapy (DAPT). (B) Safety outcome: risk of minor bleeding between TAPT versus DAPT. (C) Safety outcome: risk of any bleeding between TAPT versus DAPT. (D) Safety outcome: risk of drug discontinuation due to adverse effects between TAPT versus DAPT.
Continued
Discussion
In patients undergoing PCI, TAPT using cilostazol results in significant decrease in platelet reactivity and reduced risk of HTPR. TAPT resulted in significantly lower mean PRU, greater platelet inhibition and reduced risk of HTPR in the setting of DAPT with both standard-dose and high-dose clopidogrel. In addition, TAPT was associated with a significant reduction in CV events, including reduction in MACE, driven largely by significant reductions in TLR and TVR. Most importantly, there was a significant lower stent thrombosis with TAPT versus DAPT. Moreover, the reduction of restenosis with TAPT remained even when the analysis was restricted to trials using DES. In addition, there was numerically higher bleeding with TAPT versus DAPT, although this did not reach statistical significant. However, there was a significant increase in the risk of drug discontinuation due to adverse effects when compared with DAPT.
Platelet reactivity and outcomes
Prior studies have shown a relationship between on-treatment platelet reactivity and adverse CV events in patients undergoing PCI. In an analysis of individual patient data from six studies with 3059 patients, for every 10 U increase in PRU there was a 4% increase in primary endpoint rate of death, MI or stent thrombosis (HR 1.04; 95% CI 1.03 to 1.06; p<0.0001).17 A recent consensus statement recommended a cut-off of PRU >235 U as the threshold for identifying patients with HTPR who may be at high risk for ischaemic or thrombotic events following PCI.13 Patients with HTPR have been shown to have an increased risk of death (110% increase), MI (104% increase) and stent thrombosis (211% increase).17 ,18
Although platelet reactivity is a surrogate marker, given the wide interindividual variability in clopidogrel-induced platelet inhibition,1–3 various strategies have been tested to improve platelet inhibition. These strategies have utilised higher loading and maintenance doses of clopidogrel, or next-generation P2Y12 inhibitors such as prasugrel and ticagrelor, which are more potent that clopidogrel and have a more uniform antiplatelet effect. Doubling of the clopidogrel dose (150 mg) has been shown to significantly reduce PRU in patients with HTPR.19–21 Similarly, data from the next-generation P2Y12 inhibitors such as prasugrel and ticagrelor have shown improved platelet reactivity indices when compared with clopidogrel.22 Although the newer agents prasugrel and ticagrelor reduce MACE in randomised trials, these agents increase bleeding in patients with PCI and cost significantly more than generic clopidogrel.23 ,24
Cilostazol, a phosphodiesterase III inhibitor, exhibits antiplatelet effects by increasing cAMP within platelets, and is available as a generic drug. Our results show a significant benefit of TAPT with cilostazol in improving platelet reactivity indices in patients undergoing PCI, with lower PRU, greater platelet inhibition and a significant reduction in the risk of HTPR regardless of comparison with either standard-dose or high-dose clopidogrel. In addition, these results were seen even in comparison with DAPT using high-dose clopidogrel. Given that generic clopidogrel is now available, many clinicians opt to prescribe high-dose clopidogrel to address HTPR in patients who cannot afford newer antiplatelet agents. The results of the present study show that TAPT with cilostazol is superior even to DAPT with high-dose clopidogrel. Despite these promising results, a number of limitations must be acknowledged. Although platelet reactivity is a risk factor/surrogate marker for adverse CV events, clinical studies have not yet demonstrated that a pharmacological treatment strategy based on platelet reactivity improves outcomes.20 ,25 In the ARCTIC trial of 2440 patients randomised to platelet-function monitoring and drug adjustment group versus conventional strategy of no monitoring and drug adjustment, there were no differences in composite of death, MI, stent thrombosis, stroke, or urgent revascularisation at 1 year between the two groups, calling into question the utility of adjusting therapies based on platelet function monitoring.25
However, because cilostazol inhibits both platelet activation and smooth muscle proliferation, it has the potential to target two dreaded complications of PCI—stent thrombosis and restenosis. TAPT may reduce MACE by two or more cellular mechanisms.8–11 Our study shows significant reduction in both stent thrombosis and restenosis using TAPT with cilostazol, even in patients treated with DES. This is a potential advantage for this agent, as no antiplatelet agent, including prasugrel or ticagrelor, has been shown to have any antirestenosis property.
Therefore, a strategy of using TAPT with cilostazol has several advantages: (1) it improves the surrogate outcome of platelet reactivity relative to DAPT, including high-dose clopidogrel; (2) the antismooth muscle proliferative properties of cilostazol may make it an excellent agent to prevent restenosis resulting in reduced TVR even in patients treated with a DES; (3) the improvement in platelet reactivity indices translate into significant reduction in stent thrombosis and (4) the medication is available generically and is therefore less expensive than newer antiplatelet therapy. Thus, when used following PCI, TAPT with cilostazol has the potential to be a cost-effective therapy to improve clinical outcomes by reducing thrombotic events and restenosis. The results of this study therefore call for a randomised trial comparing a strategy of TAPT with DAPT using newer antiplatelet agents.
Our results differ from the studies of Jang et al26 and Sakurai et al27 in that these studies did not evaluate platelet reactivity outcomes and had far fewer trials than the current analysis. In our analysis, TAPT was associated with significant increase in drug discontinuation. The most commonly listed causes for drug discontinuation were headache, skin rash and palpitations/tachycardia. Sakurai et al27 similarly found a significant increase in rash and gastrointestinal side effects with TAPT.
Study limitations
As in other meta-analyses without individual patient data, we were unable to adjust for dosages of medication used or with compliance with assigned therapies. Given heterogeneity in the study protocols, clinically relevant differences could have been missed and are best assessed in a meta-analysis of individual patient data. Stroke would have been interesting to examine, as there is some evidence that cilostazol reduces stroke.28 All of the trials did not report all of the outcomes. The subgroup analyses might suffer from multiple testing. In addition, the results need to be confirmed in an ethnically diverse population, as most of the trials were done in Asian populations. However, the CREST and the OPTIMUS-2 trials, performed mainly in a non-Asian population, showed similar efficacy of cilostazol when compared with controls. The individual trials did not provide sufficient data to stratify analyses by early versus newer generation DES.
Conclusions
In patients undergoing PCI, TAPT with cilostazol is associated with significantly improved platelet reactivity indices, even when compared with DAPT with high-dose clopidogrel, and is associated with significant reduction in CV events, including reduction in BMS and DES restenosis and stent thrombosis. The dual properties of antiplatelet and antiproliferative action, the availability as a generic medication combined with the above data makes TAPT with aspirin, clopidogrel and cilostazol an attractive and strong competitor for newer antiplatelet regimens and should be evaluated in future trials.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online appendix
Footnotes
-
Contributors SB was involved in study concept and design, analysis and interpretation of the data, statistical analysis, and study supervision and also takes responsibility for the integrity of the data and the accuracy of the data analysis. SB and AS were involved in acquisition of the data and drafting of the manuscript. SB, AS, JJD, KC, FF and DLB were involved in critical revision of the manuscript for important intellectual content.
-
Funding This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
-
Competing interests DLB: Advisory Board: Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Get With The Guidelines Steering Committee; Data Monitoring Committees: Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Population Health Research Institute; Honoraria: American College of Cardiology (Editor, Clinical Trials, Cardiosource), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), Journal of the American College of Cardiology (Section Editor, Pharmacology); Research Grants: Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Roche, Sanofi Aventis, The Medicines Company; Unfunded Research: FlowCo, PLx Pharma, Takeda.
-
Provenance and peer review Not commissioned; externally peer reviewed.
-
Data sharing statement No additional data are available.